Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice
- PMID: 12975459
- PMCID: PMC2194209
- DOI: 10.1084/jem.20030837
Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice
Abstract
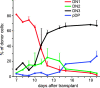
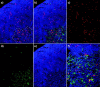
Upon thymus entry, thymic-homing progenitors undergo distinct phases of differentiation as they migrate through the cortex to the capsule, suggesting that the signals that induce these differentiation steps may be stratified in corresponding cortical regions. To better define these regions, we transplanted purified stem cells into nonirradiated congenic recipients and followed their differentiation with respect to both tissue location and time. The earliest progenitors (DN1) remained confined to a very narrow region of the cortex for about the first 10 d of intrathymic residence; this region virtually overlaps the sites of thymic entry, suggesting that DN1 cells move very little during this lengthy period of proliferation and lineage commitment. Movement out of this region into the deeper cortex is asynchronous, and corresponds to the appearance of DN2 cells. Differentiation to the DN3 stage correlates with movement across the midpoint of the cortex, indicating that stromal signals that induce functions such as TCR gene rearrangement reside mainly in the outer half of the cortex. The minimum time to reach the capsule, and thus transit to the DP stage, is approximately 13 d, with the average time a few days longer. These findings reveal for the first time the kinetics of steady-state progenitor differentiation in the thymus, as well as defining the boundaries of cortical regions that support different phases of the differentiation process. We also show that the first lineage-positive progeny of transplanted stem cells to appear in the thymus are dendritic cells in the medulla, suggesting that each new wave of new T cell production is preceded by a wave of regulatory cells that home to the medulla and ensure efficient tolerance and selection.
Figures

References
-
- Kondo, M., I.L. Weissman, and K. Akashi. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91:661–672. - PubMed
-
- Manz, M.G., D. Traver, T. Miyamoto, I.L. Weissman, and K. Akashi. 2001. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 97:3333–3341. - PubMed
-
- Mori, S., K. Shortman, and L. Wu. 2001. Characterization of thymus-seeding precursor cells from mouse bone marrow. Blood. 98:696–704. - PubMed
-
- Antica, M., L. Wu, K. Shortman, and R. Scollay. 1994. Thymic stem cells in mouse bone marrow. Blood. 84:111–117. - PubMed
-
- Michie, A.M., J.R. Carlyle, T.M. Schmitt, B. Ljutic, S.K. Cho, Q. Fong, and J.C. Zuniga-Pflucker. 2000. Clonal characterization of a bipotent T cell and NK cell progenitor in the mouse fetal thymus. J. Immunol. 164:1730–1733. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

