JunD protects against chronic kidney disease by regulating paracrine mitogens
- PMID: 12975469
- PMCID: PMC193664
- DOI: 10.1172/JCI17647
JunD protects against chronic kidney disease by regulating paracrine mitogens
Abstract
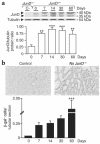
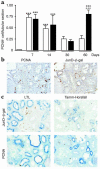
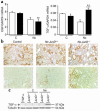
The AP-1 transcription factor, composed of Jun and Fos proteins, plays a crucial role in the fine tuning of cell proliferation. We showed previously that AP-1 complexes are activated during the proliferative response that parallels the development of renal lesions after nephron reduction, but little is known about the specific role of individual Jun/Fos components in the deterioration process. Here we used JunD knockout (JunD-/-) mice and an experimental model of chronic renal injury (75% nephron reduction) to explore the role of JunD. Nephron reduction resulted in an initial compensatory growth phase that did not require JunD. JunD, however, was essential to inhibit a second wave of cell proliferation and to halt the development of severe glomerular sclerosis, tubular dilation, and interstitial fibrosis. We show that the effects of junD inactivation are not cell autonomous and involve upregulation of the paracrine mitogen, TGF-alpha. Expression of a transgene (REM) encoding a dominant negative isoform of the EGFR, the receptor for TGF-alpha, prevented the second wave of cell proliferation and the development of renal lesions in bitransgenic JunD-/-/REM mice. We propose that JunD is part of a regulatory network that controls proliferation to prevent pathological progression in chronic renal diseases.
Figures


References
-
- Hostetter TH. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 1995;57:263–278. - PubMed
-
- Terzi F, et al. Subtotal but not unilateral nephrectomy induces hyperplasia and protooncogene expression. Am. J. Physiol. 1995;268:F793–F801. - PubMed
-
- Kliem V, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–678. - PubMed
-
- Terzi F, et al. Sodium restriction decreases AP-1 activation after nephron reduction in the rat: role in the progression of renal lesions. Exp. Nephrol. 2000;8:104–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

