CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis
- PMID: 12975472
- PMCID: PMC193672
- DOI: 10.1172/JCI19112
CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis
Abstract
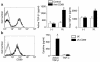
CD69 is induced after activation of leukocytes at inflammatory sites, but its physiological role during inflammation remains unknown. We explored the role of CD69 in autoimmune reactivity by analyzing a model of collagen-induced arthritis (CIA) in WT and CD69-deficient mice. CD69-/- mice showed higher incidence and severity of CIA, with exacerbated T and B cell immune responses to type II collagen. Levels of TGF-beta1 and TGF-beta2, which act as protective agents in CIA, were reduced in CD69-/- mice inflammatory foci, correlating with the increase in the proinflammatory cytokines IL-1beta and RANTES. Local injection of blocking anti-TGF-beta antibodies increased CIA severity and proinflammatory cytokine mRNA levels in CD69+/+ but not in CD69-/- mice. Moreover, in vitro engagement of CD69 induced total and active TGF-beta1 production in Concanavalin A-activated splenocyte subsets, mouse and human synovial leukocytes, and Jurkat stable transfectants of human CD69 but not in the parental CD69 negative cell line. Our results show that CD69 is a negative modulator of autoimmune reactivity and inflammation through the synthesis of TGF-beta, a cytokine that in turn downregulates the production of various proinflammatory mediators.
Figures

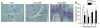

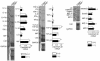



Similar articles
-
CD69: an unexpected regulator of TH17 cell-driven inflammatory responses.Sci Signal. 2011 Mar 22;4(165):pe14. doi: 10.1126/scisignal.2001825. Sci Signal. 2011. PMID: 21427408
-
CD69 targeting differentially affects the course of collagen-induced arthritis.J Leukoc Biol. 2006 Dec;80(6):1233-41. doi: 10.1189/jlb.1205749. Epub 2006 Aug 18. J Leukoc Biol. 2006. PMID: 16921025
-
The role of CD69 in acute neutrophil-mediated inflammation.Eur J Immunol. 2006 Oct;36(10):2632-8. doi: 10.1002/eji.200636355. Eur J Immunol. 2006. PMID: 16983725
-
CD69: from activation marker to metabolic gatekeeper.Eur J Immunol. 2017 Jun;47(6):946-953. doi: 10.1002/eji.201646837. Eur J Immunol. 2017. PMID: 28475283 Free PMC article. Review.
-
The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases.J Autoimmun. 2000 Feb;14(1):23-42. doi: 10.1006/jaut.1999.0339. J Autoimmun. 2000. PMID: 10648114 Review.
Cited by
-
Cold-inducible RNA-binding protein activates splenic T cells during sepsis in a TLR4-dependent manner.Cell Mol Immunol. 2018 Jan;15(1):38-47. doi: 10.1038/cmi.2016.43. Epub 2016 Aug 29. Cell Mol Immunol. 2018. PMID: 27569563 Free PMC article.
-
miRNA Expression and Interaction with Genes Involved in Susceptibility to Pristane-Induced Arthritis.J Immunol Res. 2018 Dec 16;2018:1928405. doi: 10.1155/2018/1928405. eCollection 2018. J Immunol Res. 2018. PMID: 30648118 Free PMC article.
-
Trichostatin differentially regulates Th1 and Th2 responses and alleviates rheumatoid arthritis in mice.J Clin Immunol. 2011 Jun;31(3):395-405. doi: 10.1007/s10875-011-9508-8. Epub 2011 Feb 9. J Clin Immunol. 2011. PMID: 21305388
-
Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycin.Respir Res. 2011 Oct 5;12(1):131. doi: 10.1186/1465-9921-12-131. Respir Res. 2011. PMID: 21970554 Free PMC article.
-
HDAC6 Deletion Decreases Pristane-induced Inflammation.Immunohorizons. 2024 Sep 1;8(9):668-678. doi: 10.4049/immunohorizons.2400028. Immunohorizons. 2024. PMID: 39259207 Free PMC article.
References
-
- Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol. Today. 1994;15:479–483. - PubMed
-
- Sancho D, Yanez-Mo M, Tejedor R, Sanchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium: role of lymphocyte function antigen- 1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–896. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

