Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells
- PMID: 12975473
- PMCID: PMC193661
- DOI: 10.1172/JCI15483
Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells
Abstract
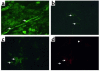
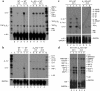
Activation of A2A adenosine receptors (A2ARs) protects kidneys from ischemia-reperfusion injury (IRI). A2ARs are expressed on bone marrow-derived (BM-derived) cells and renal smooth muscle, epithelial, and endothelial cells. To measure the contribution of A2ARs on BM-derived cells in suppressing renal IRI, we examined the effects of a selective agonist of A2ARs, ATL146e, in chimeric mice in which BM was ablated by lethal radiation and reconstituted with donor BM cells derived from GFP, A2AR-KO, or WT mice to produce GFP-->WT, A2A-KO-->WT, or WT-->WT mouse chimera. We found little or no repopulation of renal vascular endothelial cells by donor BM with or without renal IRI. ATL146e had no effect on IRI in A2A-KO mice or A2A-KO-->WT chimera, but reduced the rise in plasma creatinine from IRI by 75% in WT mice and by 60% in WT-->WT chimera. ATL146e reduced the induction of IL-6, IL-1beta, IL-1ra, and TGF-alpha mRNA in WT-->WT mice but not in A2A-KO-->WT mice. Plasma creatinine was significantly greater in A2A-KO than in WT mice after IRI, suggesting some renal protection by endogenous adenosine. We conclude that protection from renal IRI by A2AR agonists or endogenous adenosine requires activation of receptors expressed on BM-derived cells.
Figures

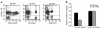
References
-
- Lauriat S, Linas SL. The role of neutrophils in acute renal failure. Semin. Nephrol. 1998;18:498–504. - PubMed
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. - PubMed
-
- Rabb H, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2000;279:F525–F531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

