Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity
- PMID: 12975475
- PMCID: PMC193666
- DOI: 10.1172/JCI18337
Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity
Abstract
To detect and characterize autoreactive T cells in diabetes-prone NOD mice, we have developed a multimeric MHC reagent with high affinity for the BDC-2.5 T cell receptor, which is reactive against a pancreatic autoantigen. A distinct population of T cells is detected in NOD mice that recognizes the same MHC/peptide target. These T cells are positively selected in the thymus at a surprisingly high frequency and exported to the periphery. They are activated specifically in the pancreatic LNs, demonstrating an autoimmune specificity that recapitulates that of the BDC-2.5 cell. These phenomena are also observed in mouse lines that share with NOD the H-2g7 MHC haplotype but carry diabetes-resistance background genes. Thus, a susceptible haplotype at the MHC seems to be the only element required for the selection and emergence of autoreactive T cells, without requiring other diabetogenic loci from the NOD genome.
Figures

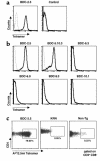

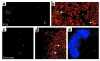

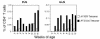
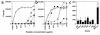

Comment in
-
Tracking autoimmune T cells in diabetes.J Clin Invest. 2003 Sep;112(6):826-8. doi: 10.1172/JCI19842. J Clin Invest. 2003. PMID: 12975466 Free PMC article.
References
-
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. - PubMed
-
- Vyse TJ, Todd JA. Genetic analysis of autoimmune diabetes. Cell. 1996;85:311–318. - PubMed
-
- Wicker LS, Todd JA, Peterson L. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 1995;13:179–200. - PubMed
-
- Hattori M, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

