Rapid Site-Directed Mutagenesis Using Two-PCR-Generated DNA Fragments Reproducing the Plasmid Template
- PMID: 12975535
- PMCID: PMC400210
- DOI: 10.1155/S1110724303209141
Rapid Site-Directed Mutagenesis Using Two-PCR-Generated DNA Fragments Reproducing the Plasmid Template
Abstract
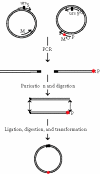
We describe a new rapid and efficient polymerase chain reaction (PCR)-based site-directed mutagenesis method. This procedure is effective with any plasmid and it employs four oligonucleotide primers. One primer contains the desired mutation, the second is oriented in the opposite direction (one of these two primers should be phosphorylated), and the third and fourth should be coding in complementary fashion for a unique restriction site to be introduced in a nonessential region. The method consists of two simultaneous PCR reactions; the PCR products are digested with the enzyme that recognizes the newly introduced unique restriction site and then ligased and used to transform competent bacteria. Additionally, the use of Dpn I facilitates the elimination of template DNA. The newly introduced restriction site is essential for ligation in the correct orientation of the two-PCR products and is further used for mutant screening. Resulting plasmids carry both the new restriction site and the desired mutation. Using this method, more than 20 mutants have already been generated (using two different kinds of templates); all these mutants were sequenced for the desired mutation and transfected into AtT-20 cells and the expressed mutant proteins encoded by the vector were assayed.
Figures




Similar articles
-
Site-directed mutagenesis of virtually any plasmid by eliminating a unique site.Anal Biochem. 1992 Jan;200(1):81-8. doi: 10.1016/0003-2697(92)90280-k. Anal Biochem. 1992. PMID: 1595905
-
A rapid method for site-specific mutagenesis using larger plasmids as templates.Biotechniques. 1993 Oct;15(4):700-4. Biotechniques. 1993. PMID: 8251172
-
An Efficient Approach for Two Distal Point Site-Directed Mutagenesis from Randomly Ligated PCR Products.Appl Biochem Biotechnol. 2019 Dec;189(4):1318-1326. doi: 10.1007/s12010-019-03059-1. Epub 2019 Jul 1. Appl Biochem Biotechnol. 2019. PMID: 31264104
-
Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis.Biotechniques. 1992 Aug;13(2):214-20. Biotechniques. 1992. PMID: 1327007
-
Polishing the craft of genetic diversity creation in directed evolution.Biotechnol Adv. 2013 Dec;31(8):1707-21. doi: 10.1016/j.biotechadv.2013.08.021. Epub 2013 Sep 6. Biotechnol Adv. 2013. PMID: 24012599 Review.
Cited by
-
USP21 regulates Hippo pathway activity by mediating MARK protein turnover.Oncotarget. 2017 Jul 18;8(38):64095-64105. doi: 10.18632/oncotarget.19322. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28969054 Free PMC article.
-
The tomato leucine-rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity.PLoS One. 2014 Mar 27;9(3):e93302. doi: 10.1371/journal.pone.0093302. eCollection 2014. PLoS One. 2014. PMID: 24675749 Free PMC article.
-
DUB3 Deubiquitylating Enzymes Regulate Hippo Pathway Activity by Regulating the Stability of ITCH, LATS and AMOT Proteins.PLoS One. 2017 Jan 6;12(1):e0169587. doi: 10.1371/journal.pone.0169587. eCollection 2017. PLoS One. 2017. PMID: 28061504 Free PMC article.
-
Characterization of the signaling domain of the NO-responsive regulator NorR from Ralstonia eutropha H16 by site-directed mutagenesis.J Bacteriol. 2007 Apr;189(7):2743-9. doi: 10.1128/JB.01865-06. Epub 2007 Feb 2. J Bacteriol. 2007. PMID: 17277050 Free PMC article.
-
In vivo cross-linking of EpsG to EpsL suggests a role for EpsL as an ATPase-pseudopilin coupling protein in the Type II secretion system of Vibrio cholerae.Mol Microbiol. 2011 Feb;79(3):786-98. doi: 10.1111/j.1365-2958.2010.07487.x. Mol Microbiol. 2011. PMID: 21255118 Free PMC article.
References
-
- Smith M. In vitro mutagenesis. Annu Rev Genet. 1985;19:423–462. - PubMed
-
- Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. - PubMed
-
- Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51–59. - PubMed
-
- Ge L, Rudolph P. Simultaneous introduction of multiple mutations using overlap extension PCR. Biotechniques. 1997;22(1):28–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

