Drosophila free-running rhythms require intercellular communication
- PMID: 12975658
- PMCID: PMC193604
- DOI: 10.1371/journal.pbio.0000013
Drosophila free-running rhythms require intercellular communication
Abstract
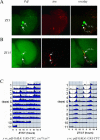
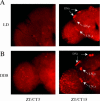
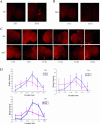
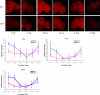
Robust self-sustained oscillations are a ubiquitous characteristic of circadian rhythms. These include Drosophila locomotor activity rhythms, which persist for weeks in constant darkness (DD). Yet the molecular oscillations that underlie circadian rhythms damp rapidly in many Drosophila tissues. Although much progress has been made in understanding the biochemical and cellular basis of circadian rhythms, the mechanisms that underlie the differences between damped and self-sustaining oscillations remain largely unknown. A small cluster of neurons in adult Drosophila brain, the ventral lateral neurons (LN(v)s), is essential for self-sustained behavioral rhythms and has been proposed to be the primary pacemaker for locomotor activity rhythms. With an LN(v)-specific driver, we restricted functional clocks to these neurons and showed that they are not sufficient to drive circadian locomotor activity rhythms. Also contrary to expectation, we found that all brain clock neurons manifest robust circadian oscillations of timeless and cryptochrome RNA for many days in DD. This persistent molecular rhythm requires pigment-dispersing factor (PDF), an LN(v)-specific neuropeptide, because the molecular oscillations are gradually lost when Pdf(01) mutant flies are exposed to free-running conditions. This observation precisely parallels the previously reported effect on behavioral rhythms of the Pdf(01) mutant. PDF is likely to affect some clock neurons directly, since the peptide appears to bind to the surface of many clock neurons, including the LN(v)s themselves. We showed that the brain circadian clock in Drosophila is clearly distinguishable from the eyes and other rapidly damping peripheral tissues, as it sustains robust molecular oscillations in DD. At the same time, different clock neurons are likely to work cooperatively within the brain, because the LN(v)s alone are insufficient to support the circadian program. Based on the damping results with Pdf(01) mutant flies, we propose that LN(v)s, and specifically the PDF neuropeptide that it synthesizes, are important in coordinating a circadian cellular network within the brain. The cooperative function of this network appears to be necessary for maintaining robust molecular oscillations in DD and is the basis of sustained circadian locomotor activity rhythms.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
References
-
- Allada R, White N, So W, Hall J, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless . Cell. 1998;93:791–804. - PubMed
-
- Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: The genetics of fly and mouse circadian clocks. Ann Rev Neurosci. 2001;24:1091–1119. - PubMed
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

