LAX and SPA: major regulators of shoot branching in rice
- PMID: 13130077
- PMCID: PMC208832
- DOI: 10.1073/pnas.1932414100
LAX and SPA: major regulators of shoot branching in rice
Abstract
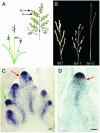
The aerial architecture of plants is determined primarily by the pattern of shoot branching. Although shoot apical meristem initiation during embryogenesis has been extensively studied by molecular genetic approaches using Arabidopsis, little is known about the genetic mechanisms controlling axillary meristem initiation, mainly because of the insufficient number of mutants that specifically alter it. We identified the LAX PANICLE (LAX) and SMALL PANICLE (SPA) genes as the main regulators of axillary meristem formation in rice. LAX encodes a basic helix-loop-helix transcription factor and is expressed in the boundary between the shoot apical meristem and the region of new meristem formation. This pattern of LAX expression was repeatedly observed in every axillary meristem, consistent with our observation that LAX is involved in the formation of all types of axillary meristems throughout the ontogeny of a rice plant. Ectopic LAX expression in rice caused pleiotropic effects, including dwarfing, an altered pattern of stem elongation, darker color, bending of the lamina joint, absence of the midribs of leaves, and severe sterility.
Figures



References
-
- Grbri', V. (2001) in Meristematic Tissues in Plant Growth and Development, eds. McManus, M. T. & Veit, B. E. (Sheffield Academic, Sheffield, U.K.), pp. 142–171.
-
- Stirnberg, P., vande Sande, K. & Leyser, H. M. O. (2002) Development (Cambridge, U.K.) 129, 1131–1141. - PubMed
-
- Doebley, J., Stec, A. & Hubbard, L. (1997) Nature 386, 485–488. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

