Nonreplicative homologous RNA recombination: promiscuous joining of RNA pieces?
- PMID: 13130136
- PMCID: PMC1370486
- DOI: 10.1261/rna.5111803
Nonreplicative homologous RNA recombination: promiscuous joining of RNA pieces?
Abstract
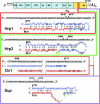
Biologically important joining of RNA pieces in cells, as exemplified by splicing and some classes of RNA editing, is posttranscriptional, whereas in RNA viruses it is generally believed to occur during viral RNA polymerase-dependent RNA synthesis. Here, we demonstrate the assembly of precise genome of an RNA virus (poliovirus) from its cotransfected fragments, which does not require specific RNA sequences, takes place before generation of the viral RNA polymerase, and occurs in different ways: Apparently unrestricted ligation of the terminal nucleotides, joining of any one of the two entire fragments with the relevant internal nucleotide of its partner, or internal crossovers within the overlapping sequence. Incorporation of the entire 5' or 3' partners into the recombinant RNA is activated by the presence of terminal 3'-phosphate and 5'-OH, respectively. Such postreplicative reactions, fundamentally differing from the known site-specific and structurally demanding cellular RNA rearrangements, might contribute to the origin and evolution of RNA viruses and could generate new RNA species during all stages of biological evolution.
Figures





References
-
- Abelson, J., Trotta, C.R., and Li, H. 1998. tRNA splicing. J. Biol. Chem. 273: 12685–12688. - PubMed
-
- Agol, V.I. 1997. Recombination and other genomic rearrangements in picornaviruses. Semin. Virol. 8: 1–9.
-
- Agol, V.I. 2002. Picornavirus genetics: An overview. In The molecular biology of picornaviruses (eds. B.L. Semler and E. Wimmer), pp. 269–284. ASM Press, Washington, DC.
-
- Arnold, J.J. and Cameron, C.E. 1999. Poliovirus RNA-dependent RNA polymerase (3Dpol) is sufficient for template switching in vitro. J. Biol. Chem. 274: 2706–2716. - PubMed
-
- Avota, E., Berzins, V., Grens, E., Vishnevsky, Y., Luce, R., and Biebricher, C. 1998. The natural 6 S RNA found in Qβ-infected cells is derived from host and phage RNA. J. Mol. Biol. 276: 7–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
