Evidence for the implication of phosphoinositol signal transduction in mu-opioid inhibition of DNA synthesis
- PMID: 1322969
- PMCID: PMC2571949
- DOI: 10.1111/j.1471-4159.1992.tb08357.x
Evidence for the implication of phosphoinositol signal transduction in mu-opioid inhibition of DNA synthesis
Abstract
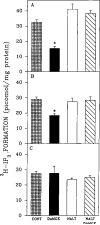
An opioid receptor agonist, [D-Ala2,Me-Phe4,Glyol5]enkephalin (DAMGE), decreased [3H]thymidine incorporation into DNA of fetal rat brain cell aggregates. This action proved to depend on the dose of this enkephalin analog and the interval the aggregates were maintained in culture. The opioid antagonist naltrexone and the mu-specific antagonist cyclic D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr amide (CTOP) reversed the DAMGE effect, arguing for a receptor-mediated mechanism. The mu-opioid nature of this receptor was further established by inhibiting DNA synthesis with the highly mu-selective agonist morphiceptin and blocking its action with CTOP. Several other opioids, pertussis toxin, and LiCl also diminished DNA synthesis, whereas cholera toxin elicited a modest increase. Naltrexone completely reversed the inhibition elicited by the combination of DAMGE and low doses of LiCl but not by that of high levels of LiCl alone. The enkephalin analog also reduced basal [3H]inositol trisphosphate and glutamate-stimulated [3H]inositol monophosphate and [3H]inositol bisphosphate accumulation in the aggregates. These DAMGE effects were reversed by naltrexone and were temporally correlated with the inhibition of DNA synthesis. A selective protein kinase C inhibitor, chelerythrine, also inhibited thymidine incorporation dose-dependently. The effect of DAMGE was not additive in the presence of chelerythrine but appeared to be consistent with their actions being mediated via a common signaling pathway. These results suggest the involvement of the phosphoinositol signal transduction system in the modulation of thymidine incorporation into DNA by DAMGE.
Figures







References
-
- Ashkenazi A, Ramachandran J, Capon DJ. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989;340:146–150. - PubMed
-
- Barg J, Levy R, Simantov RJ. Paradoxical and subtype-specific effects of opiate antagonists on the expression of opioid receptors in rat brain cultures. J. Neurosci. Res. 1989a;22:322–330. - PubMed
-
- Barg J, Levy R, Simantov R. Expression of the three opioid receptor subtypes μ, δ and κ in guinea pig and rat brain cell cultures and in vivo. Int. J. Dev. Neurosci. 1989b;7:173–180. - PubMed
-
- Barg J, Belcheva M, Bern WT, Lambourne B, McLachlan JA, Tolman KC, Johnson FE, Coscia CJ. Desipramine modulation of sigma and opioid peptide receptor expression in glial cells. Peptides. 1991;12:845–849. - PubMed
-
- Bartolome JV, Bartolome MB, Daltner LA, Evans CJ, Barchas JD, Kuhn CM, Schanberg SM. Effects of β-endorphin on ornithine decarboxylase in tissues of developing rats. A potential role for this endogenous neuropeptide in the modulation of tissue growth. Life Sci. 1986;38:2355–2362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

