Tissue-specific expression of the skeletal alpha-actin gene involves sequences that can function independently of MyoD and Id
- PMID: 1333317
- PMCID: PMC6057380
Tissue-specific expression of the skeletal alpha-actin gene involves sequences that can function independently of MyoD and Id
Abstract
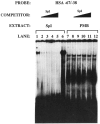
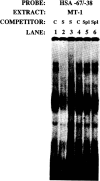
The skeletal alpha-actin gene is a member of the sarcomeric contractile protein gene family and is specifically expressed in differentiated muscle. The skeletal alpha-actin gene is regulated efficiently by enhancer and regulatory sequences between nucleotide positions -1282 and -87. In the present study we have shown that the sequences 3' of nucleotide position -87 can functionally interact with the SV40 enhancer in a tissue-specific manner and can restrict the ubiquitous function of the SV40 enhancer to myogenic cells. Site-specific cassette mutagenesis was used to delimit the sequences upstream of the TATA motif (-32), between nucleotide positions -64 and -37, that mediate efficient expression in myogenic cells in the presence of the SV40 enhancer. The skeletal alpha-actin promoter was trans-activated by the helix-loop-helix (HLH) transcription factors MyoD, MRF-4, and Myogenin, in pluripotential 10T1/2 fibroblasts and trans-repressed by the HLH protein Id (inhibitor of differentiation) in myogenic C2C12 cells. This trans-regulation required sequences upstream of -87 and occurred independently of the two consensus E boxes (CANNTG) at positions +18 and +71. The -64/-37 region interacted with purified Sp1 and an unidentified protein(s), proximal regulatory factor(s) I (PRF-I). We conclude that the muscle-specific expression of the skeletal alpha-actin promoter is not simply determined by MyoD elements and enhancer and regulatory sequences, but that the minimal promoter contains important determinants of cell-specific transcription that can function independently of the helix-loop-helix transcription factors.
Figures






Similar articles
-
Heterodimers of myogenic helix-loop-helix regulatory factors and E12 bind a complex element governing myogenic induction of the avian cardiac alpha-actin promoter.Mol Cell Biol. 1991 May;11(5):2439-50. doi: 10.1128/mcb.11.5.2439-2450.1991. Mol Cell Biol. 1991. PMID: 1850096 Free PMC article.
-
Muscle-specific expression of the troponin I gene requires interactions between helix-loop-helix muscle regulatory factors and ubiquitous transcription factors.Mol Cell Biol. 1991 Jan;11(1):267-80. doi: 10.1128/mcb.11.1.267-280.1991. Mol Cell Biol. 1991. PMID: 1846022 Free PMC article.
-
MyoD and myogenin act on the chicken myosin light-chain 1 gene as distinct transcriptional factors.Mol Cell Biol. 1993 Nov;13(11):7153-62. doi: 10.1128/mcb.13.11.7153-7162.1993. Mol Cell Biol. 1993. PMID: 8413304 Free PMC article.
-
A novel myogenic regulatory circuit controls slow/cardiac troponin C gene transcription in skeletal muscle.Mol Cell Biol. 1994 Mar;14(3):1870-85. doi: 10.1128/mcb.14.3.1870-1885.1994. Mol Cell Biol. 1994. PMID: 8114720 Free PMC article.
-
Activation of muscle-specific transcription by myogenic helix-loop-helix proteins.Symp Soc Exp Biol. 1992;46:331-41. Symp Soc Exp Biol. 1992. PMID: 1341046 Review.
Cited by
-
Phosphorylation of MRF4 transactivation domain by p38 mediates repression of specific myogenic genes.EMBO J. 2004 Jan 28;23(2):365-75. doi: 10.1038/sj.emboj.7600056. Epub 2004 Jan 22. EMBO J. 2004. PMID: 14739931 Free PMC article.
-
Rho/Rho-associated kinase signal regulates myogenic differentiation via myocardin-related transcription factor-A/Smad-dependent transcription of the Id3 gene.J Biol Chem. 2008 Jul 25;283(30):21230-41. doi: 10.1074/jbc.M710525200. Epub 2008 May 12. J Biol Chem. 2008. PMID: 18477564 Free PMC article.
-
Activation of myoD gene transcription by 3,5,3'-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors.Nucleic Acids Res. 1994 Feb 25;22(4):583-91. doi: 10.1093/nar/22.4.583. Nucleic Acids Res. 1994. PMID: 8127707 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
