The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes
- PMID: 13679511
- PMCID: PMC284793
- DOI: 10.1091/mbc.e03-06-0387
The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes
Abstract
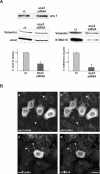
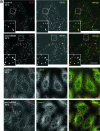
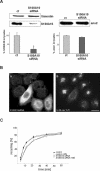
The Ca2+- and lipid-binding protein annexin 2, which resides in a tight heterotetrameric complex with the S100 protein S100A10 (p11), has been implicated in the structural organization and dynamics of endosomal membranes. To elucidate the function of annexin 2 and S100A10 in endosome organization and trafficking, we used RNA-mediated interference to specifically suppress annexin 2 and S100A10 expression. Down-regulation of both proteins perturbed the distribution of transferrin receptor- and rab11-positive recycling endosomes but did not affect uptake into sorting endosomes. The phenotype was highly specific and could be rescued by reexpression of the N-terminal annexin 2 domain or S100A10 in annexin 2- or S100A10-depleted cells, respectively. Whole-mount immunoelectron microscopy of the aberrantly localized recycling endosomes in annexin 2/S100A10 down-regulated cells revealed extensively bent tubules and an increased number of endosome-associated clathrin-positive buds. Despite these morphological alterations, the kinetics of transferrin uptake and recycling was not affected to a significant extent, indicating that the proper positioning of recycling endosomes is not a rate-limiting step in transferrin recycling. The phenotype generated by this transient loss-of-protein approach shows for the first time that the annexin 2/S100A10 complex functions in the intracellular positioning of recycling endosomes and that both subunits are required for this activity.
Figures





Similar articles
-
The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport.PLoS One. 2007 Oct 31;2(10):e1118. doi: 10.1371/journal.pone.0001118. PLoS One. 2007. PMID: 17971878 Free PMC article.
-
Association of annexin 2 with recycling endosomes requires either calcium- or cholesterol-stabilized membrane domains.Eur J Cell Biol. 2001 Aug;80(8):499-507. doi: 10.1078/0171-9335-00184. Eur J Cell Biol. 2001. PMID: 11561901
-
Alix regulates cortical actin and the spatial distribution of endosomes.J Cell Sci. 2005 Jun 15;118(Pt 12):2625-35. doi: 10.1242/jcs.02382. Epub 2005 May 24. J Cell Sci. 2005. PMID: 15914539
-
S100A10/p11: family, friends and functions.Pflugers Arch. 2008 Jan;455(4):575-82. doi: 10.1007/s00424-007-0313-4. Epub 2007 Jul 19. Pflugers Arch. 2008. PMID: 17638009 Review.
-
S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors.Front Biosci. 2005 Jan 1;10:300-25. doi: 10.2741/1529. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574370 Review.
Cited by
-
The formation of the cAMP/protein kinase A-dependent annexin 2-S100A10 complex with cystic fibrosis conductance regulator protein (CFTR) regulates CFTR channel function.Mol Biol Cell. 2007 Sep;18(9):3388-97. doi: 10.1091/mbc.e07-02-0126. Epub 2007 Jun 20. Mol Biol Cell. 2007. PMID: 17581860 Free PMC article.
-
Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells.Infect Immun. 2007 Aug;75(8):3925-34. doi: 10.1128/IAI.00106-07. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502389 Free PMC article.
-
Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4,5) bisphosphate-containing lipid rafts and increases viral production in 293T cells.PLoS One. 2009;4(3):e5020. doi: 10.1371/journal.pone.0005020. Epub 2009 Mar 27. PLoS One. 2009. PMID: 19325895 Free PMC article.
-
Acute Administration of Branched-Chain Amino Acids Increases the Pro-BDNF/Total-BDNF Ratio in the Rat Brain.Neurochem Res. 2015 May;40(5):885-93. doi: 10.1007/s11064-015-1541-1. Epub 2015 Feb 14. Neurochem Res. 2015. PMID: 25681161
-
The Essential Role of anxA2 in Langerhans Cell Birbeck Granules Formation.Cells. 2020 Apr 15;9(4):974. doi: 10.3390/cells9040974. Cells. 2020. PMID: 32326440 Free PMC article.
References
-
- Drust, D.S., and Creutz, C.E. (1988). Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature 331, 88–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

