The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells
- PMID: 13679516
- PMCID: PMC307554
- DOI: 10.1091/mbc.e03-05-0338
The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells
Abstract
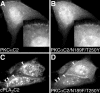
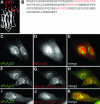
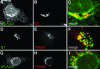
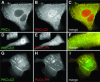
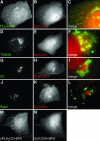
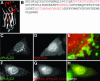
Translocation of cytosolic phospholipase A2 (cPLA2) to Golgi and ER in response to intracellular calcium mobilization is regulated by its calcium-dependent lipid-binding, or C2, domain. Although well studied in vitro, the biochemical characteristics of the cPLA2C2 domain offer no predictive value in determining its intracellular targeting. To understand the molecular basis for cPLA2C2 targeting in vivo, the intracellular targets of the synaptotagmin 1 C2A (Syt1C2A) and protein kinase Calpha C2 (PKCalphaC2) domains were identified in Madin-Darby canine kidney cells and compared with that of hybrid C2 domains containing the calcium binding loops from cPLA2C2 on Syt1C2A and PKCalphaC2 domain backbones. In response to an intracellular calcium increase, PKCalphaC2 targeted plasma membrane regions rich in phosphatidylinositol-4,5-bisphosphate, and Syt1C2A displayed a biphasic targeting pattern, first targeting phosphatidylinositol-4,5-bisphosphate-rich regions in the plasma membrane and then the trans-Golgi network. In contrast, the Syt1C2A/cPLA2C2 and PKCalphaC2/cPLA2C2 hybrids targeted Golgi/ER and colocalized with cPLA2C2. The electrostatic properties of these hybrids suggested that the membrane binding mechanism was similar to cPLA2C2, but not PKCalphaC2 or Syt1C2A. These results suggest that primarily calcium binding loops 1 and 3 encode structural information specifying Golgi/ER targeting of cPLA2C2 and the hybrid domains.
Figures




References
-
- Arcaro, A., Volinia, S., Zvelebil, M.J., Stein, R., Watton, S.J., Layton, M.J., Gout, I., Ahmadi, K., Downward, J., and Waterfield, M.D. (1998). Human phosphoinositide 3-kinase C2 beta, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem. 273, 33082-33090. - PubMed
-
- Balla, T., and Varnai, P. (2002). Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Science's Stke: signal transduction knowledge environment. PL3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

