Measuring stem cell frequency in epidermis: a quantitative in vivo functional assay for long-term repopulating cells
- PMID: 13679571
- PMCID: PMC208771
- DOI: 10.1073/pnas.2034935100
Measuring stem cell frequency in epidermis: a quantitative in vivo functional assay for long-term repopulating cells
Abstract
Epidermal stem cells play a central role in tissue homeostasis, wound repair, tumor initiation, and gene therapy. A major impediment to the purification and molecular characterization of epidermal stem cells is the lack of a quantitative assay for cells capable of long-term repopulation in vivo, such as exists for hematopoietic cells. The tremendous strides made in the characterization and purification of hematopoietic stem cells have been critically dependent on the availability of competitive transplantation assays, because these assays permit the accurate quantitation of long-term repopulating cells in vivo. We have developed an analogous functional assay for epidermal stem cells, and have measured the frequency of functional epidermal stem cells in interfollicular epidermis. These studies indicate that cells capable of long-term reconstitution of a squamous epithelium reside in the interfollicular epidermis. We find that the frequency of these long-term repopulating cells is 1 in 35,000 total epidermal cells, or in the order of 1 in 104 basal epidermal cells, similar to that of hematopoietic stem cells in the bone marrow, and much lower than previously estimated in epidermis. Furthermore, these studies establish a novel functional assay that can be used to validate immunophenotypic markers and enrichment strategies for epidermal stem cells, and to quantify epidermal stem cells in various keratinocyte populations. Thus further studies using this type of assay for epidermis should aid in the progress of cutaneous stem cell-targeted gene therapy, and in more basic studies of epidermal stem cell regulation and differentiation.
Figures
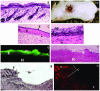
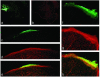
References
-
- Morris, R. J., Fischer, S. M. & Slaga, T. J. (1985) J. Invest. Dermatol. 84, 277–281. - PubMed
-
- Mackenzie, I. C. & Bickenbach, J. R. (1985) Cell Tissue Res. 242, 551–556. - PubMed
-
- Niemann, C. & Watt, F. M. (2002) Trends Cell Biol. 12, 185–192. - PubMed
-
- Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

