Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors
- PMID: 13679574
- PMCID: PMC208770
- DOI: 10.1073/pnas.1936664100
Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors
Abstract
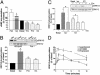
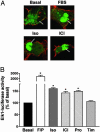
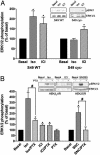
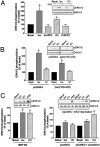
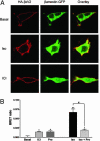
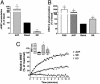
It is becoming increasingly clear that signaling via G protein-coupled receptors is a diverse phenomenon involving receptor interaction with a variety of signaling partners. Despite this diversity, receptor ligands are commonly classified only according to their ability to modify G protein-dependent signaling. Here we show that beta2AR ligands like ICI118551 and propranolol, which are inverse agonists for Gs-stimulated adenylyl cyclase, induce partial agonist responses for the mitogen-activated protein kinases extracellular signal-regulated kinase (ERK) 1/2 thus behaving as dual efficacy ligands. ERK1/2 activation by dual efficacy ligands was not affected by ADP-ribosylation of Galphai and could be observed in S49-cyc- cells lacking Galphas indicating that, unlike the conventional agonist isoproterenol, these drugs induce ERK1/2 activation in a Gs/i-independent manner. In contrast, this activation was inhibited by a dominant negative mutant of beta-arrestin and was abolished in mouse embryonic fibroblasts lacking beta-arrestin 1 and 2. The role of beta-arrestin was further confirmed by showing that transfection of beta-arrestin 2 in these knockout cells restored ICI118551 promoted ERK1/2 activation. ICI118551 and propranolol also promoted beta-arrestin recruitment to the receptor. Taken together, these observations suggest that beta-arrestin recruitment is not an exclusive property of agonists, and that ligands classically classified as inverse agonists rely exclusively on beta-arrestin for their positive signaling activity. This phenomenon is not unique to beta2-adrenergic ligands because SR121463B, an inverse agonist on the V2 vasopressin receptor-stimulated adenylyl cyclase, recruited beta-arrestin and stimulated ERK1/2. These results point to a multistate model of receptor activation in which ligand-specific conformations are capable of differentially activating distinct signaling partners.
Figures
References
-
- Gutkind, J. S. (2000) Sci. STKE 40, RE1. - PubMed
-
- Schmitt, J. M. & Stork, P. J. (2000) J. Biol. Chem. 275, 25342–25350. - PubMed
-
- Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655–661. - PubMed
-
- McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J. & Lefkowitz, R. J. (2000) Science 290, 1574–1577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

