ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3
- PMID: 13679578
- PMCID: PMC208793
- DOI: 10.1073/pnas.1933593100
ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3
Abstract
Covalent modifications of histone tails modulate gene expression via chromatin organization. As examples, methylation of lysine 9 residues of histone H3 (H3) (H3-K9) is believed to repress transcription by compacting chromatin, whereas methylation of lysine 4 residues of H3 (H3-K4) is believed to activate transcription by relaxing chromatin. The Drosophila trithorax group protein absent, small, or homeotic discs 1 (ASH1) is involved in maintaining active transcription of many genes. Here we report that in extreme ash1 mutants, no H3-K4 methylation is detectable. Within the limits of our assays, this lack of detectable H3-K4 methylation implies that ASH1 is required for essentially all H3-K4 methylation that occurs in vivo. We report further that the 149-aa SET domain of ASH1 is sufficient for H3-K4 methylation in vitro. These findings support a model in which ASH1 is directly involved in maintaining active transcription by conferring a relaxed chromatin structure.
Figures
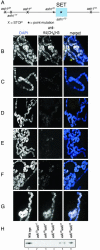
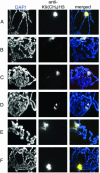
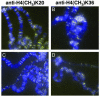
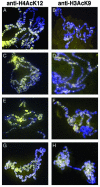

References
-
- Strahl, B. D. & Allis, C. D. (2000) Nature 403, 1–45. - PubMed
-
- Murray, K. (1964) Biochemistry 3, 10–15. - PubMed
-
- Rea, S., Eisenhaber, F., O'Carroll, D., Strahl, B. D., Sun, Z., Schmid, M., Opravil, S., Ponting, C. P., Allis, C. D. & Jenuwein, T. (2000) Nature 406, 593–599. - PubMed
-
- Beisel, C., Imhof, A., Greene, J., Kremmer, E. & Sauer, F. (2002) Nature 419, 857–862. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

