Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release
- PMID: 13679582
- PMCID: PMC208826
- DOI: 10.1073/pnas.1834314100
Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release
Erratum in
- Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15285
Abstract
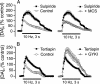
In many cells, ATP-sensitive K+ channels (KATP channels) couple metabolic state to excitability. In pancreatic beta cells, for example, this coupling regulates insulin release. Although KATP channels are abundantly expressed in the brain, their physiological role and the factors that regulate them are poorly understood. One potential regulator is H2O2. We reported previously that dopamine (DA) release in the striatum is modulated by endogenous H2O2, generated downstream from glutamatergic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptor activation. Here we investigated whether H2O2-sensitive KATP channels contribute to DA-release modulation by glutamate and gamma-aminobutyric acid (GABA). This question is important because DA-glutamate interactions underlie brain functions, including motor control and cognition. Synaptic DA release was evoked by using local electrical stimulation in slices of guinea pig striatum and monitored in real time with carbon-fiber microelectrodes and fast-scan cyclic voltammetry. The KATP-channel antagonist glibenclamide abolished the H2O2-dependent increase in DA release usually seen with AMPA-receptor blockade by GYKI-52466 [1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride] and the decrease in DA release seen with GABA-type-A-receptor blockade by picrotoxin. In contrast, 5-hydroxydecanoate, a mitochondrial KATP-channel blocker, was ineffective, as were sulpiride, a D2-receptor antagonist, and tertiapin, a G protein-coupled K+-channel inhibitor. Diazoxide, a sulfonylurea receptor 1 (SUR1)selective KATP-channel opener, prevented DA modulation by H2O2, glutamate, and GABA, whereas cromakalim, a SUR2-selective opener, did not. Thus, endogenous H2O2 activates SUR1-containing KATP channels in the plasma membrane to inhibit DA release. These data not only demonstrate that KATP channels can modulate CNS transmitter release in response to fast-synaptic transmission but also introduce H2O2 as a KATP-channel regulator.
Figures

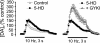

References
-
- Noma, A. (1983) Nature 305, 147–148. - PubMed
-
- Cook, D. L. & Hales, C. N. (1984) Nature 311, 271–273. - PubMed
-
- Ashcroft, S. J. & Ashcroft, F. M. (1990) Cell. Signalling 2, 197–214. - PubMed
-
- Liss, B. & Roeper, J. (2001) News Physiol. Sci. 16, 214–217. - PubMed
-
- Cook, D. L., Satin, L. S., Ashford, M. L. & Hales, C. N. (1988) Diabetes 37, 495–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

