The BOLD response and the gamma oscillations respond differently than evoked potentials: an interleaved EEG-fMRI study
- PMID: 14499000
- PMCID: PMC222904
- DOI: 10.1186/1471-2202-4-22
The BOLD response and the gamma oscillations respond differently than evoked potentials: an interleaved EEG-fMRI study
Abstract
Background: The integration of EEG and fMRI is attractive because of their complementary precision regarding time and space. But the relationship between the indirect hemodynamic fMRI signal and the more direct EEG signal is uncertain. Event-related EEG responses can be analyzed in two different ways, reflecting two different kinds of brain activity: evoked, i.e. phase-locked to the stimulus, such as evoked potentials, or induced, i.e. non phase-locked to the stimulus such as event-related oscillations. In order to determine which kind of EEG activity was more closely related with fMRI, EEG and fMRI signals were acquired together, while subjects were presented with two kinds of rare events intermingled with frequent distractors. Target events had to be signaled by pressing a button and Novel events had to be ignored.
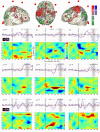
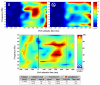
Results: Both Targets and Novels triggered a P300, of larger amplitude in the Novel condition. On the opposite, the fMRI BOLD response was stronger in the Target condition. EEG event-related oscillations in the gamma band (32-38 Hz) reacted in a way similar to the BOLD response.
Conclusions: The reasons for such opposite differential reactivity between oscillations / fMRI on the one hand, and evoked potentials on the other, are discussed in the paper. Those results provide further arguments for a closer relationship between fast oscillations and the BOLD signal, than between evoked potentials and the BOLD signal.
Figures

References
-
- Aine CJ. A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/FMRI and PET. Crit Rev Neurobiol. 1995;9:229–309. - PubMed
-
- Matsuura T, Kanno I. Quantitative and temporal relationship between local cerebral blood flow and neuronal activation induced by somatosensory stimulation in rats. Neurosci Res. 2001;40:281–290. - PubMed
-
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

