Interleukin-18 facilitates the early antimicrobial host response to Escherichia coli peritonitis
- PMID: 14500466
- PMCID: PMC201063
- DOI: 10.1128/IAI.71.10.5488-5497.2003
Interleukin-18 facilitates the early antimicrobial host response to Escherichia coli peritonitis
Abstract
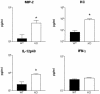
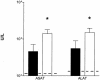
To determine the role of endogenous interleukin-18 (IL-18) during peritonitis, IL-18 gene-deficient (IL-18 KO) mice and wild-type mice were intraperitoneally (i.p.) infected with Escherichia coli, the most common causative agent found in septic peritonitis. Peritonitis was associated with a bacterial dose-dependent increase in IL-18 concentrations in peritoneal fluid and plasma. After infection, IL-18 KO mice had significantly more bacteria in the peritoneal lavage fluid and were more susceptible for progression to systemic infection at 6 and 20 h postinoculation than wild-type mice. The relative inability of IL-18 KO mice to clear E. coli from the abdominal cavity was not due to an intrinsic defect in the phagocytosing capacity of their peritoneal macrophages or neutrophils. IL-18 KO mice displayed an increased neutrophil influx into the peritoneal cavity, but these migratory neutrophils were less activate, as reflected by a reduced CD11b surface expression. These data suggest that endogenous IL-18 plays an important role in the early antibacterial host response during E. coli-induced peritonitis.
Figures
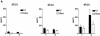
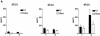







References
-
- Akira, S. 2000. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12:59-63. - PubMed
-
- Akira, S. 2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:1-56. - PubMed
-
- Arndt, P. G., G. Fantuzzi, and E. Abraham. 2000. Expression of interleukin-18 in the lung after endotoxemia or hemorrhage-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 22:708-713. - PubMed
-
- Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. - PubMed
-
- Bosscha, K., K. Reijnders, P. F. Hulstaert, A. Algra, and C. van der Werken. 1997. Prognostic scoring systems to predict outcome in peritonitis and intra-abdominal sepsis. Br. J. Surg. 84:1532-1534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

