Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro
- PMID: 14500475
- PMCID: PMC201046
- DOI: 10.1128/IAI.71.10.5565-5575.2003
Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro
Abstract
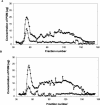
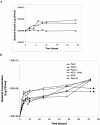
Pseudomonas aeruginosa has emerged as an important causative agent of bacterial keratitis, a rapidly progressive ocular condition that may result in blindness. Secretory mucin forms the main constituent of the precorneal tear film, a three-layer film on the ocular surface protecting the underlying corneal epithelium from potential pathogens. The purpose of the present study was to compare mucin degradation mechanisms between ocular P. aeruginosa strains. Mucin degradation was assessed by agarose electrophoresis, lectin blotting, and size exclusion chromatography. The results indicate that certain P. aeruginosa strains (Paer12, ATCC 15442, 6294, and Paer25) had depleted mucin from the culture supernatant and that this was contingent on the inherent ability of these isolates to produce proteases. Non-protease-producing strains (Paer1 and Paer3) did not appreciably degrade mucin. Further, galactosidase, N-acetylglucosaminidase, and N-acetylgalactosaminidase activities were detected in some strains, suggesting the operation of further mechanisms of mucin degradation by P. aeruginosa. Mucin degradation by P. aeruginosa also seemed to be for the acquisition of nutrients, as a growth advantage was observed in mucin-depleting strains over nondepleting strains in the long term. It is postulated that the degradation of mucin serves to collapse the mucin barrier and its associated network containing antibacterial tear components and to provide energy for sustained bacterial growth.
Figures




References
-
- Argüeso, P., and I. K. Gipson. 2001. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp. Eye Res. 73:281-289. - PubMed
-
- Beighton, D., K. Smith, D. A. Glenister, K. Salamon, and C. W. Keevil. 1988. Increased degradative enzyme production by dental plaque bacteria in mucin-limited continuous culture. Microb. Ecol. Health Dis. 1:85-94.
-
- Berry, M., R. B. Ellingham, and A. P. Corfield. 1996. Polydispersity of normal human conjunctival mucins. Investig. Ophthalmol. Visual Sci. 37:2559-2571. - PubMed
-
- Bradshaw, D. J., K. A. Homer, P. D. Marsh, and D. Beighton. 1994. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140:3407-3412. - PubMed
-
- Byrd, J. C., P. Yan, L. Sternberg, C. K. Yunker, J. M. Scheiman, and R. S. Bresalier. 1997. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology 113:455-464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

