Activation of human dendritic cells is modulated by components of the outer membranes of Neisseria meningitidis
- PMID: 14500478
- PMCID: PMC201071
- DOI: 10.1128/IAI.71.10.5590-5597.2003
Activation of human dendritic cells is modulated by components of the outer membranes of Neisseria meningitidis
Abstract
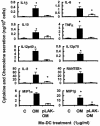
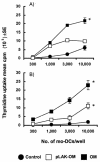
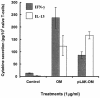
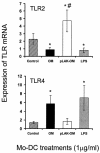
Neisseria meningitidis serogroup B is a major cause of life-threatening meningitis and septicemia worldwide, and no effective vaccine is available. Initiation of innate and acquired immune responses to N. meningitidis is likely to be dependent on cellular responses of dendritic cells (DC) to antigens present in the outer membrane (OM) of the meningococcus. In this study, the responses of human monocyte-derived DC (mo-DC) to OM isolated from parent (lipopolysaccharide [LPS]-replete) meningococci and from a mutant deficient in LPS were investigated. Parent OM selectively up-regulated Toll-like receptor 4 (TLR4) mRNA expression and induced mo-DC maturation, as reflected by increased production of chemokines, proinflammatory cytokines, and CD83, CD80, CD86, CD40, and major histocompatibility complex (MHC) class II molecules. In contrast, LPS-deficient OM selectively up-regulated TLR2 mRNA expression and induced moderate increases in both cytokine production and expression of CD86 and MHC class II molecules. Preexposure to OM, with or without LPS, augmented the allostimulatory properties of mo-DC, which induced proliferation of naive CD4+ CD45RA+ T cells. In addition, LPS-replete OM induced a greater gamma interferon/interleukin-13 ratio in naive T cells, whereas LPS-deficient OM induced the reverse profile. These data demonstrate that components of the OM, other than LPS, are also likely to be involved in determining the levels of DC activation and the nature of the T-helper immune response.
Figures

References
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Bjerre, A., B. Brusletto, E. Rosenqvist, E. Namork, P. Kierulf, R. Ovstebo, G. B. Joo, and P. Brandtzaeg. 2000. Cellular activating properties and morphology of membrane-bound and purified meningococcal lipopolysaccharide. J. Endotoxin Res. 6:437-445. - PubMed
-
- Brandtzaeg, P., A. Bjerre, R. Ovstebo, B. Brusletto, G. B. Joo, and P. Kierulf. 2001. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin Res. 7:401-420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

