Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans
- PMID: 14500501
- PMCID: PMC201089
- DOI: 10.1128/IAI.71.10.5794-5802.2003
Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans
Abstract
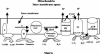
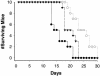
We identified a homologue of the alternative oxidase gene in a screen to identify genes that are preferentially transcribed in response to a shift to 37 degrees C in the human-pathogenic yeast Cryptococcus neoformans. Alternative oxidases are nucleus-encoded mitochondrial proteins that have two putative roles: they can function in parallel with the classic cytochrome oxidative pathway to produce ATP, and they may counter oxidative stress within the mitochondria. The C. neoformans alternative oxidase gene (AOX1) was found to exist as a single copy in the genome, and it encodes a putative protein of 401 amino acids. An aox1 mutant strain was created using targeted gene disruption, and the mutant strain was reconstituted to wild type using a full-length AOX1. Compared to both the wild-type and reconstituted strains, the aox1 mutant strain was not temperature sensitive but did have significant impairment of both respiration and growth when treated with inhibitors of the classic cytochrome oxidative pathway. The aox1 mutant strain was also found to be more sensitive to the oxidative stressor tert-butyl hydroperoxide. The aox1 mutant strain was significantly less virulent than both the wild type and the reconstituted strain in the murine inhalational model, and it also had significantly impaired growth within a macrophage-like cell line. These data demonstrate that the alternative oxidase of C. neoformans can make a significant contribution to metabolism, has a role in the yeast's defense against exogenous oxidative stress, and contributes to the virulence composite of this organism, possibly by improving survival within phagocytic cells.
Figures
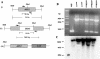



References
-
- Albury, M. S., C. Affourtit, and A. L. Moore. 1998. A highly conserved glutamate residue (Glu-270) is essential for plant alternative oxidase activity. J. Biol. Chem. 273:30301-30305. - PubMed
-
- Aubert, S., R. Bligny, D. A. Day, J. Whelan, and R. Douce. 1997. Induction of alternative oxidase synthesis by herbicides inhibiting branched-chain amino acid synthesis. Plant J. 11:649-657.
-
- Barja, G. 1999. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 31:347-366. - PubMed
-
- Borghouts, C., C. Q. Scheckhuber, O. Stephan, and H. D. Osiewacz. 2002. Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PACtr3 encoding a copper transporter. Int. J. Biochem. Cell Biol. 34:1355-1371. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

