M1 protein triggers a phosphoinositide cascade for group A Streptococcus invasion of epithelial cells
- PMID: 14500504
- PMCID: PMC201040
- DOI: 10.1128/IAI.71.10.5823-5830.2003
M1 protein triggers a phosphoinositide cascade for group A Streptococcus invasion of epithelial cells
Abstract
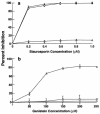
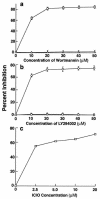
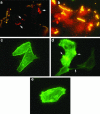
Invasion of nonphagocytic cells by bacteria provides a favorable niche for persistence and evasion of host defenses and antibiotics. M protein is a major virulence factor because it promotes high-frequency invasion of epithelial cells by group A Streptococcus (GAS) and also renders the bacterium resistant to phagocytosis. In this study, we investigated the role of M1 protein from serotype M1 strain 90-226 in regulating mammalian signal transduction and cytoskeletal rearrangement for bacterial entry. LY294002 and wortmannin, which are inhibitors of phosphatidylinositol 3-kinase (PI 3-K) blocked invasion of epithelial cells by GAS by 75 and 80%, respectively, but failed to inhibit invasion by Salmonella enterica serovar Typhimurium. Also, epithelial cells transiently transfected with dominant negative p85 and p110 genes, the regulatory and catalytic subunits of PI 3-K, respectively, were less able to be invaded by GAS. To separate the influence of other streptococcal virulence factors from M protein, Lactococcus lactis was engineered to express M1 protein on its surface. L. lactis(pLM1) invaded epithelial cells efficiently in vitro, and PI 3-K inhibitors blocked 90% of this invasion. Purified soluble M1 protein stimulated the formation of stress fibers and actin tuffs on epithelial cells. LY294002 and wortmannin inhibited these cellular changes. A phosphoinositide analogue also inhibited the invasion of epithelial cells by GAS. Therefore, M1 protein, either directly or via bound fibronectin, initiates signals that depend on the lipid kinase PI 3-K pathway, which paves the way for cytoskeletal rearrangement that internalize the bacterium.
Figures
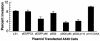
Similar articles
-
Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells.Infect Immun. 1998 Oct;66(10):4593-601. doi: 10.1128/IAI.66.10.4593-4601.1998. Infect Immun. 1998. PMID: 9746555 Free PMC article.
-
Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion.Microb Pathog. 2001 Nov;31(5):231-42. doi: 10.1006/mpat.2001.0467. Microb Pathog. 2001. PMID: 11710843
-
A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin alpha 5beta 1-fibronectin-M1 protein complexes.Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2858-63. doi: 10.1073/pnas.050587897. Proc Natl Acad Sci U S A. 2000. PMID: 10706638 Free PMC article.
-
The nonideal coiled coil of M protein and its multifarious functions in pathogenesis.Adv Exp Med Biol. 2011;715:197-211. doi: 10.1007/978-94-007-0940-9_12. Adv Exp Med Biol. 2011. PMID: 21557065 Free PMC article. Review.
-
[Mechanisms of immune evasion by Streptococcus pyogenes].Tanpakushitsu Kakusan Koso. 2009 Jun;54(8 Suppl):982-7. Tanpakushitsu Kakusan Koso. 2009. PMID: 21089528 Review. Japanese. No abstract available.
Cited by
-
NLRX1 Negatively Regulates Group A Streptococcus Invasion and Autophagy Induction by Interacting With the Beclin 1-UVRAG Complex.Front Cell Infect Microbiol. 2018 Nov 14;8:403. doi: 10.3389/fcimb.2018.00403. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30488027 Free PMC article.
-
Insights gained from sequencing Australian non-invasive and invasive Streptococcus pyogenes isolates.Microb Genom. 2024 Jan;10(1):001152. doi: 10.1099/mgen.0.001152. Microb Genom. 2024. PMID: 38197886 Free PMC article.
-
Interplay between group A Streptococcus and host innate immune responses.Microbiol Mol Biol Rev. 2024 Mar 27;88(1):e0005222. doi: 10.1128/mmbr.00052-22. Epub 2024 Mar 7. Microbiol Mol Biol Rev. 2024. PMID: 38451081 Free PMC article. Review.
-
Salmonella enterica serovar typhimurium invades fibroblasts by multiple routes differing from the entry into epithelial cells.Infect Immun. 2010 Jun;78(6):2700-13. doi: 10.1128/IAI.01389-09. Epub 2010 Apr 5. Infect Immun. 2010. PMID: 20368348 Free PMC article.
-
Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for PspC-mediated internalization of pneumococci by respiratory epithelial cells.J Biol Chem. 2009 Jul 17;284(29):19427-36. doi: 10.1074/jbc.M109.003442. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473971 Free PMC article.
References
-
- Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infection of skin and soft tissues. N. Engl. J. Med. 334:240-245. - PubMed
-
- Cantrell, D. A. 2001. Phosphoinositide 3-kinase signaling pathways. J. Cell Sci. 114:1439-1445. - PubMed
-
- Chan, T. O., U. Rodeck, A. M. Chan, A. Kimmelman, S. E. Rittenhouse, G. Panayotou, and N. Tischlis. 2002. Small GTPase and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell 1:181-191. - PubMed
-
- Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

