An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells
- PMID: 14500514
- PMCID: PMC201066
- DOI: 10.1128/IAI.71.10.5940-5950.2003
An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells
Abstract
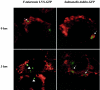
The facultative intracellular bacterium Francisella tularensis is a highly virulent and contagious organism, and little is known about its intracellular survival mechanisms. We studied the intracellular localization of the attenuated human vaccine strain, F. tularensis LVS, in adherent mouse peritoneal cells, in mouse macrophage-like cell line J774A.1, and in human macrophage cell line THP-1. Confocal microscopy of infected J774A.1 cells indicated that during the first hour of infection the bacteria colocalized with the late endosomal-lysosomal glycoprotein LAMP-1, but within 3 h this colocalization decreased significantly from approximately 60% to 30%. Transmission electron microscopy revealed that >90% of bacteria were not enclosed by a phagosomal membrane after 2 h of infection, and some bacteria were in vacuoles that were only partially surrounded by a limiting membrane. Similar findings were obtained with all three host cell types. Immunoelectron microscopy performed with an F. tularensis LVS-specific polyclonal rabbit antiserum showed that the antiserum stained a thick, evenly distributed capsule-like material in bacteria grown in broth. In contrast, intracellular F. tularensis LVS cells were only marginally stained with this antiserum. Instead, most of the immunoreactive material was diffusely localized in the phagosomes or was associated with the phagosomal membrane. Our findings indicate that F. tularensis LVS is able to escape from the phagosomes of macrophages via a mechanism that may involve degradation of the phagosomal membrane.
Figures






References
-
- Alvarez-Dominguez, C., R. Roberts, and P. D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731-743. - PubMed
-
- Amer, A. O., and M. S. Swanson. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5:56-61. - PubMed
-
- Anthony, L. S., P. J. Morrissey, and F. E. Nano. 1992. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J. Immunol. 148:1829-1834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

