Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation
- PMID: 14500772
- PMCID: PMC2343575
- DOI: 10.1113/jphysiol.2003.050419
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation
Abstract
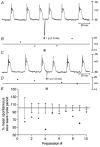
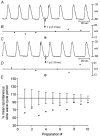
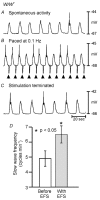
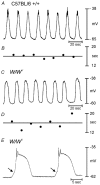
Phase advancement of electrical slow waves and regulation of pacemaker frequency was investigated in the circular muscle layer of the gastric antra of wild-type and W/W(V) mice. Slow waves in the murine antrum of wild-type animals had an intrinsic frequency of 4.4 cycles min(-1) and were phase advanced and entrained to a maximum of 6.3 cycles min(-1) using 0.1 ms pulses of electrical field stimulation (EFS) (three pulses delivered at 3-30 Hz). Pacing of slow waves was blocked by tetrodotoxin (TTX) and atropine, suggesting phase advancement was mediated via intrinsic cholinergic nerves. Phase advancement and entrainment of slow waves via this mechanism was absent in W/W(V) mutants which lack intramuscular interstitial cells of Cajal (ICC-IM). These data suggest that neural regulation of slow wave frequency and regulation of smooth muscle responses to slow waves are mediated via nerve-ICC-IM interactions. With longer stimulation parameters (1.0-2.0 ms), EFS phase advanced and entrained slow waves in wild-type and W/W(V) animals. Pacing with 1-2 ms pulses was not inhibited by TTX or atropine. These data suggest that stimulation with longer pulse duration is capable of directly activating the pacemaker mechanism in ICC-MY networks. In summary, intrinsic excitatory neurons can phase advance and increase the frequency of antral slow waves. This form of regulation is mediated via ICC-IM. Longer pulse stimulation can directly activate ICC-MY in the absence of ICC-IM.
Figures





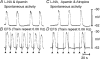

References
-
- Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979;282:415–417. - PubMed
-
- Bortolotti M. The ‘electrical way’ to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883. - PubMed
-
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

