The sources and sequestration of Ca(2+) contributing to neuroeffector Ca(2+) transients in the mouse vas deferens
- PMID: 14500773
- PMCID: PMC2343581
- DOI: 10.1113/jphysiol.2003.049734
The sources and sequestration of Ca(2+) contributing to neuroeffector Ca(2+) transients in the mouse vas deferens
Abstract
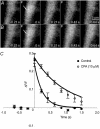
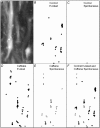
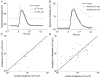
The detection of focal Ca(2+) transients (called neuroeffector Ca(2+) transients, or NCTs) in smooth muscle of the mouse isolated vas deferens has been used to detect the packeted release of ATP from nerve terminal varicosities acting at postjunctional P2X receptors. The present study investigates the sources and sequestration of Ca(2+) in NCTs. Smooth muscle cells in whole mouse deferens were loaded with the Ca(2+) indicator Oregon Green 488 BAPTA-1 AM and viewed with a confocal microscope. Ryanodine (10 microM) decreased the amplitude of NCTs by 45 +/- 6 %. Cyclopiazonic acid slowed the recovery of NCTs (from a time course of 200 +/- 10 ms to 800 +/- 100 ms). Caffeine (3 mM) induced spontaneous focal smooth muscle Ca(2+) transients (sparks). Neither of the T-type Ca(2+) channel blockers NiCl2 (50 microM) or mibefradil dihydrochloride (10 microM) affected the amplitude of excitatory junction potentials (2 +/- 5 % and -3 +/- 10 %) or NCTs (-20 +/- 36 % and 3 +/- 13 %). In about 20 % of cells, NCTs were associated with a local, subcellular twitch that remained in the presence of the alpha1-adrenoceptor antagonist prazosin (100 nM), showing that NCTs can initiate local contractions. Slow (5.8 +/- 0.4 microm s(-1)), spontaneous smooth muscle Ca(2+) waves were occasionally observed. Thus, Ca(2+) stores initially amplify and then sequester the Ca(2+) that enters through P2X receptors and there is no amplification by local voltage-gated Ca(2+) channels.
Figures




References
-
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. - PubMed
-
- Bian JH, Ghosh TK, Wang JC, Gill DL. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991;266:8801–8806. - PubMed
-
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. - PubMed
-
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

