Heterogeneity of postsynaptic receptor occupancy fluctuations among glycinergic inhibitory synapses in the zebrafish hindbrain
- PMID: 14500774
- PMCID: PMC2343629
- DOI: 10.1113/jphysiol.2003.049577
Heterogeneity of postsynaptic receptor occupancy fluctuations among glycinergic inhibitory synapses in the zebrafish hindbrain
Abstract
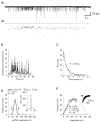
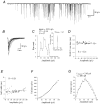
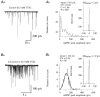
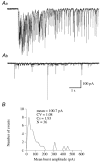
The amplitude of glycinergic miniature inhibitory postsynaptic currents (mIPSCs) varies considerably in neurons recorded in the isolated hindbrain of 50-h-old zebrafish larvae. At this age, glycinergic synapses are functionally mature. In order to measure the occupancy level of postsynaptic glycine receptors (GlyRs) and to determine the pre- and/or postsynaptic origin of its variability, we analysed mIPSCs within bursts evoked by alpha-latrotoxin (0.1-1 nM). Two types of burst were observed according to their mIPSC frequencies: 'slow' bursts with clearly spaced mIPSCs and 'fast' bursts characterised by superimposed events. Non-stationary noise analysis of mIPSCs in some 'slow' bursts recorded in the presence or in the absence of Ca2+ denoted that mIPSC amplitude variance did not depend on the quantity of neurotransmitters released (presynaptic origin), but rather on intrinsic stochastic behaviour of the same group of GlyRs (postsynaptic origin). In these bursts, the open probability measured at the peak of the mIPSCs was close to 0.5 while the maximum open probability is close to 0.9 for the synaptic isoform of GlyRs (heteromeric alpha1/beta GlyRs). In 'fast' bursts with superimposed events, a correlation was found between the amplitude of mIPSCs and the basal current level measured at their onset, which could suggest that the same group of GlyRs is activated during such bursts. Altogether, our results indicate that glycine synapses can display different release modes in the presence of alpha-latrotoxin. They also indicate that, in our model, postsynaptic GlyRs cannot be saturated by the release of a single vesicle.
Figures

References
-
- Ali DW, Drapeau P, Legendre P. Development of spontaneous glycinergic currents in the Mauthner neuron of the zebrafish embryo. J Neurophysiol. 2000;84:1726–1736. - PubMed
-
- Almers W, Tse FW. Transmitter release from synapses: does a preassembled fusion pore initiate exocytosis. Neuron. 1990;4:813–818. - PubMed
-
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. - PubMed
-
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA. α-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem. 2001;276:44695–44703. - PubMed
-
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

