A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity
- PMID: 14500783
- PMCID: PMC208803
- DOI: 10.1073/pnas.1930781100
A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity
Abstract
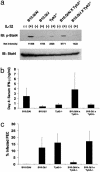
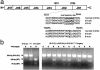
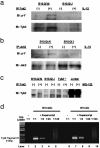
The B10.Q/J strain of mice was serendipitously discovered to be highly susceptible to infection by the intracellular protozoan parasite, Toxoplasma gondii but markedly resistant to induction of autoimmune arthritis. We have previously shown that the B10.Q/J phenotype is controlled by a single recessive locus and is associated with lymphocyte hyporesponsiveness to IL-12. Using genetic approaches, we have now localized the B10.Q/J locus to chromosome 9 and established its identity as Tyk2, a Janus kinase essential for IL-12 and IFN-alpha/beta cytokine signaling. The B10.Q/J Tyk2 gene contained a single missense mutation resulting in a nonconservative amino acid substitution (E775K) in an invariant motif of the pseudokinase (Janus kinase homology 2) domain. This mutation appeared to result in the absence of the B10.Q/J-encoded Tyk2 protein, despite presence of Tyk2-specific transcripts. Phenotypically, B10.Q/J cells were indistinguishable from Tyk2-deficient cells, showing impaired signaling and biologic responses to IL-12, IL-23, and type I IFNs. The analogous E782K mutant of human Tyk2 failed to restore IFN-alpha responsiveness in Tyk2 null 11,1 cells. Our results indicate a crucial role for Tyk2 in T helper 1-mediated protective and pathogenic immune responses. An additional implication of our findings is that naturally occurring mutations in the Tyk2 gene may underlie altered susceptibilities to infectious or autoimmune diseases in human and animal populations.
Figures

References
-
- Yap, G., Pesin, M. & Sher, A. (2000) J. Immunol. 165, 628–631. - PubMed
-
- Yap, G. S., Ortmann, R., Shevach, E. & Sher, A. (2001) J. Immunol. 166, 5720–5725. - PubMed
-
- Mattner, F., Magram, J., Ferrante, J., Launois, P., Di Padova, K., Behin, R., Gately, M. K., Louis, J. A. & Alber, G. (1996) Eur. J. Immunol. 26, 1553–1559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

