Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins
- PMID: 14500900
- PMCID: PMC208754
- DOI: 10.1073/pnas.2033520100
Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins
Abstract
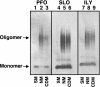
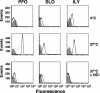
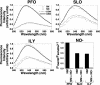
The cholesterol-dependent cytolysins (CDCs) constitute a large family of pore-forming toxins that function exclusively on cholesterol-containing membranes. A detailed analysis of the various stages in the cytolytic mechanism of three members of the CDC family revealed that significant depletion of cholesterol from the erythrocyte membrane stalls these toxins in the prepore complex. Therefore, the depletion of membrane cholesterol prevents the insertion of the transmembrane beta-barrel and pore formation. These unprecedented findings provide a paradigm for the involvement of cholesterol in the CDC cytolytic mechanism and that of other pore-forming toxins whose activity is enhanced by the presence of membrane cholesterol.
Figures


Similar articles
-
A Key Motif in the Cholesterol-Dependent Cytolysins Reveals a Large Family of Related Proteins.mBio. 2020 Sep 29;11(5):e02351-20. doi: 10.1128/mBio.02351-20. mBio. 2020. PMID: 32994330 Free PMC article.
-
The cholesterol-dependent cytolysin family of gram-positive bacterial toxins.Subcell Biochem. 2010;51:551-77. doi: 10.1007/978-90-481-8622-8_20. Subcell Biochem. 2010. PMID: 20213558 Review.
-
Perfringolysin O structure and mechanism of pore formation as a paradigm for cholesterol-dependent cytolysins.Subcell Biochem. 2014;80:63-81. doi: 10.1007/978-94-017-8881-6_5. Subcell Biochem. 2014. PMID: 24798008 Free PMC article. Review.
-
What planar lipid membranes tell us about the pore-forming activity of cholesterol-dependent cytolysins.Biophys Chem. 2013 Dec 1;182:64-70. doi: 10.1016/j.bpc.2013.06.015. Epub 2013 Jul 2. Biophys Chem. 2013. PMID: 23876488 Review.
-
The cholesterol-dependent cytolysins.Curr Top Microbiol Immunol. 2001;257:15-33. doi: 10.1007/978-3-642-56508-3_2. Curr Top Microbiol Immunol. 2001. PMID: 11417120 Review.
Cited by
-
Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis.Pathogens. 2019 Dec 30;9(1):33. doi: 10.3390/pathogens9010033. Pathogens. 2019. PMID: 31905867 Free PMC article.
-
Equinatoxin II permeabilizing activity depends on the presence of sphingomyelin and lipid phase coexistence.Biophys J. 2008 Jul;95(2):691-8. doi: 10.1529/biophysj.108.129981. Epub 2008 Apr 4. Biophys J. 2008. PMID: 18390598 Free PMC article.
-
Perfringolysin O association with ordered lipid domains: implications for transmembrane protein raft affinity.Biophys J. 2010 Nov 17;99(10):3255-63. doi: 10.1016/j.bpj.2010.09.028. Biophys J. 2010. PMID: 21081073 Free PMC article.
-
Intermedilysin cytolytic activity depends on heparan sulfates and membrane composition.PLoS Genet. 2021 Feb 12;17(2):e1009387. doi: 10.1371/journal.pgen.1009387. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33577603 Free PMC article.
-
The Cholesterol-dependent Cytolysin Membrane-binding Interface Discriminates Lipid Environments of Cholesterol to Support β-Barrel Pore Insertion.J Biol Chem. 2015 Jul 17;290(29):17733-17744. doi: 10.1074/jbc.M115.656769. Epub 2015 Jun 1. J Biol Chem. 2015. PMID: 26032415 Free PMC article.
References
-
- Heuck, A. P., Tweten, R. K. & Johnson, A. E. (2001) Biochemistry 40, 9065–9073. - PubMed
-
- Alouf, J. E. (1999) in Bacterial Toxins: A Comprehensive Sourcebook, eds. Alouf, J. & Freer, J. (Academic, London), pp. 443–456.
-
- Alouf, J. E. (2000) Int. J. Med. Microbiol. 290, 351–356. - PubMed
-
- Jacobs, T., Cima-Cabal, M. D., Darji, A., Méndez, F. J., Vázquez, F., Jacobs, A. A. C., Shimada, Y., Ohno-Iwashita, Y., Weiss, S. & de los Toyos, J. R. (1999) FEBS Lett. 459, 463–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

