Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile
- PMID: 14500901
- PMCID: PMC208837
- DOI: 10.1073/pnas.2032704100
Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile
Abstract
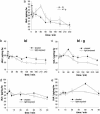
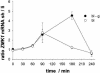
Auxin redistribution along gravistimulated maize coleoptiles causes differential expression of the auxin-induced K+-channel gene ZMK1 (Zea mays K+ channel 1) and precedes the curvature response. To evaluate the role of ZMK1 during phototropism, we here investigated blue light-stimulated coleoptiles. Four hours of blue light stimulation resulted in phototropic bending (23 degrees ). Rotation on a clinostat, at nominally "zero" gravity, and simultaneous stimulation with unidirectional blue light, however, resulted in up to 51 degrees bending toward the light. Differential ZMK1 transcription reached a maximum after 90 min of blue light stimulation under gravity, whereas ZMK1 expression remained asymmetric for at least 180 min in photostimulated coleoptiles on a clinostat. We therefore conclude that the stronger phototropic bending under nominally "zero" gravity results from prolonged differential expression of ZMK1. Under both conditions, asymmetric expression of ZMK1 could be superimposed on the lateral auxin gradient across the coleoptile tip, whereas the gene for the blue light receptor phototropin 1 (PHOT1), expressed in the tip only, was not differentially regulated in response to blue light. The activation of the two different receptors eliciting the photo- and gravitropic response of the coleoptile thus feeds into a common signaling pathway, resulting in auxin redistribution in the coleoptile tip and finally in differential transcription of ZMK1. In the process of signal integration, gravity transduction restricts the magnitude of the blue light-inducible ZMK1 gradient. The spatial and temporal distribution of ZMK1 transcripts and thus differential K+ uptake in both flanks of the coleoptile seem to limit the stimulus-induced bending of this sensory organ.
Figures
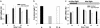
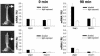
References
-
- Darwin, C. (1880) The Power of Movements in Plants (Murray, London).
-
- Went, F. W. (1928) Receuil des Travaux Botaniques Neerlandais 25, 1–116.
-
- Cholodny, N. (1928) Planta 6, 118–134.
-
- Iino, M. (1991) Plant Cell Environ. 14, 279–286.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

