Nonhuman primate parthenogenetic stem cells
- PMID: 14504386
- PMCID: PMC304106
- DOI: 10.1073/pnas.2034195100
Nonhuman primate parthenogenetic stem cells
Erratum in
- Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):693
Abstract
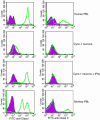
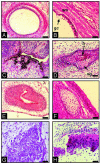
Parthenogenesis is the biological phenomenon by which embryonic development is initiated without male contribution. Whereas parthenogenesis is a common mode of reproduction in lower organisms, the mammalian parthenote fails to produce a successful pregnancy. We herein describe in vitro parthenogenetic development of monkey (Macaca fascicularis) eggs to the blastocyst stage, and their use to create a pluripotent line of stem cells. These monkey stem cells (Cyno-1 cells) are positive for telomerase activity and are immunoreactive for alkaline phosphatase, octamer-binding transcription factor 4 (Oct-4), stage-specific embryonic antigen 4 (SSEA-4), tumor rejection antigen 1-60 (TRA 1-60), and tumor rejection antigen 1-81 (TRA 1-81) (traditional markers of human embryonic stem cells). They have a normal chromosome karyotype (40 + 2) and can be maintained in vitro in an undifferentiated state for extended periods of time. Cyno-1 cells can be differentiated in vitro into dopaminergic and serotonergic neurons, contractile cardiomyocyte-like cells, smooth muscle, ciliated epithelia, and adipocytes. When Cyno-1 cells were injected into severe combined immunodeficient mice, teratomas with derivatives from all three embryonic germ layers were obtained. When grown on fibronectin/laminin-coated plates and in neural progenitor medium, Cyno-1 cells assume a neural precursor phenotype (immunoreactive for nestin). However, these cells remain proliferative and express no functional ion channels. When transferred to differentiation conditions, the nestin-positive precursors assume neuronal and epithelial morphologies. Over time, these cells acquire electrophysiological characteristics of functional neurons (appearance of tetrodotoxin-sensitive, voltage-dependent sodium channels). These results suggest that stem cells derived from the parthenogenetically activated nonhuman primate egg provide a potential source for autologous cell therapy in the female and bypass the need for creating a competent embryo.
Figures





References
-
- Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science 282, 1145-1147. - PubMed
-
- Thomson, J. A., Kalishman, J., Golos, T. G., Durning, M., Harris, C. P. & Hearn, J. P. (1996) Biol. Reprod. 55, 254-259. - PubMed
-
- Suemori, H., Tada, T., Torii, R., Hosoi, Y., Kobayashi, K., Imahie, H., Kondo, Y., Iritani, A. & Nakatsuji, N. (2001) Dev. Dyn. 222, 273-279. - PubMed
-
- Cibelli, J. B., Grant, K. A., Chapman, K. B., Cunniff, K., Worst, T., Green, H. L., Walker, S. J., Gutin, P. H., Vilner, L., Tabar, V., et al. (2002) Science 295, 819. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

