Effects of lipid-based oral formulations on plasma and tissue amphotericin B concentrations and renal toxicity in male rats
- PMID: 14506053
- PMCID: PMC201114
- DOI: 10.1128/AAC.47.10.3339-3342.2003
Effects of lipid-based oral formulations on plasma and tissue amphotericin B concentrations and renal toxicity in male rats
Abstract
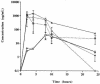
The purpose of this study was to determine the effects of various lipid and mixed-micelle formulations on the oral absorption and renal toxicity of amphotericin B (AMB) in rats. The maximum concentration of AMB in plasma and the area under the concentration-time curve for 0 to 24 h for AMB were elevated in rats administered triglyceride (TG)-rich AMB formulations in comparison to those in rats given (i) AMB preformulated as a micelle containing sodium deoxycholate with sodium phosphate as a buffer (DOC-AMB), (ii) an AMB-lipid complex suspension, or (iii) AMB solubilized in methanol. Furthermore, our findings suggest that AMB incorporated into TG-based oral formulations has less renal toxicity than DOC-AMB.
Figures
References
-
- Blom, G. 1958. Statistical estimates and transformed beta variables, p. 1-58. John Wiley and Sons, Inc., New York, N.Y.
-
- Dangi, J. S., S. P. Vyas, and V. K. Dixit. 1998. Effects of various lipid-bile salt mixed micelles on the intestinal absorption of amphotericin-B in rat. Drug Dev. Ind. Pharm. 24:631-635. - PubMed
-
- Egito, E. S. T., I. B. Araujo, P. G. Bolivar, L. Damasceno, and J. C. Price. 2002. Amphotericin B/emulsion admixture interactions: an approach concerning the reduction of amphotericin B toxicity. J. Pharm. Sci. 91:2354-2366. - PubMed
-
- Fagerholm, U., M. Johansson, and H. Lennernas. 1996. Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 13:1336-1342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

