Members of the USF family of helix-loop-helix proteins bind DNA as homo- as well as heterodimers
- PMID: 1450663
- PMCID: PMC6057381
Members of the USF family of helix-loop-helix proteins bind DNA as homo- as well as heterodimers
Abstract
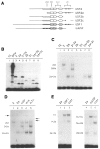
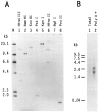
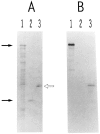
We have isolated human cDNA clones for USF2, a new member of the upstream stimulatory factor (USF) family of transcription factors. Analysis of these clones revealed the existence of highly conserved elements in the C terminal region of all USF proteins. These include the basic region, helix-loop-helix (HLH) motif, and, in the case of the human proteins, the C-terminal leucine repeat (LR). In addition, a highly conserved USF-specific domain is located immediately upstream of the basic region. Using in vitro translated proteins, we found that all members of the USF family bound DNA as dimers. The N-terminal portion of USF, including the USF-specific domain, was entirely dispensable for dimer formation and DNA-binding. However, deletion mutants of USF2 lacking the LR were deficient in DNA-binding activity. Interestingly, each of the USF proteins could form functional heterodimers with the other family members, including the sea urchin USF, which does not have a LR motif. This indicates that the conserved LR in human USF is not required for dimer formation, and influences only indirectly DNA-binding.
Figures


References
-
- Beckmann H., Su L. K., and Kadesh T. (1990), Genes Dev 4, 177–179. - PubMed
-
- Beckmann H. and Kadesh T. (1991), Genes Dev 5, 1057–1066. - PubMed
-
- Blackwell T. K., Kretzner L., Blackwood E. M., and Eisenman R. N. (1990), Science 250, 1149–1151. - PubMed
-
- Blackwood E. M. and Eisenman R. N. (1991), Science 252, 1211–1217. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
