Differential modes of activation define orphan subclasses within the steroid/thyroid receptor superfamily
- PMID: 1450665
- PMCID: PMC6057375
Differential modes of activation define orphan subclasses within the steroid/thyroid receptor superfamily
Abstract
We report that three orphan receptors, hERR1, hERR2, and hTR2, members of the steroid/thyroid receptor (SR/TR) superfamily, can be activated by different ligand-independent pathways. hERR1 and hERR2 exhibit constitutive activity in the absence of exogenously added ligands. Furthermore, this constitutive activity is localized in the carboxy terminal domain of both receptors and can be transferred to other members of this superfamily using domain switch strategies. In addition, we show that hERR1 can remain constitutively active in the less evolved eukaryotic cell Saccharomyces cerevisiae. In contrast, hTR2 is not constitutively active. However, a chimera of hTR2 can be activated in a ligand-independent manner through a signal transduction pathway initiated at the cell membrane by the neurotransmitter dopamine. Like hERR1 and hERR2, hTR2 is ligand-independently activated through its carboxy terminal domain. Together, these results suggest the existence of emerging subgroups within the SR/TR superfamily that can regulate gene expression through different modes of activation.
Figures



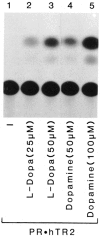
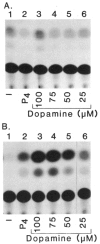
References
-
- Arriza J. L., Weinberger C., Cerelli G., Glasser T. M., Handelin B. L., Housman D. E., and Evans R. M. (1987), Science 237, 268–275. - PubMed
-
- Butt T. R., Khan M. I., Marsh J., Ecker D. J., and Crooke S. T. (1988), J Biol Chem 263, 16364–16371. - PubMed
-
- Carson M. A., Tsai M.-J., Conneely O. M., Maxwell B. L., Clark J. H., Dobson A. D. W., Elbrecht A., Toft D. O., Schrader W. T., and O’Malley B. W. (1987), Mol Endocrinol 1, 791–801. - PubMed
-
- Carson M. A., Lee A. T., Dobson A. D. W., Conneely O. M., Schrader W. T., and O’Malley B. W. (1990), J Steroid Biochem 34, 1–9. - PubMed
-
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., and Stanley P. (1986), Somat Cell Mol Genet 12, 237–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
