Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice
- PMID: 14507636
- PMCID: PMC1868298
- DOI: 10.1016/s0002-9440(10)63486-4
Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice
Abstract
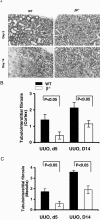
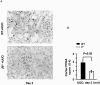
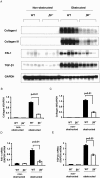
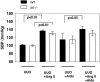
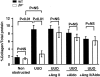
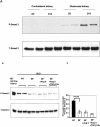
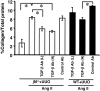
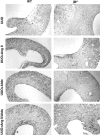
Transforming growth factor-beta1 (TGF-beta1) and the renin-angiotensin-aldosterone system are key mediators in kidney fibrosis. Integrin alphavbeta6, a heterodimeric matrix receptor expressed in epithelia, binds and activates latent TGF-beta1. We used beta6 integrin-null mice (beta6(-/-)) to determine the role of local TGF-beta1 activation in renal fibrosis in the unilateral ureteral obstruction (UUO) model. Obstructed kidneys from beta6(-/-) mice showed less injury than obstructed kidneys from wild-type (WT) mice, associated with lower collagen I, collagen III, plasminogen activator inhibitor (PAI-1), and TGF-beta1 mRNA levels and lower collagen content. Infusion with either angiotensin II (Ang II) or aldosterone (Aldo) or combination in beta6(-/-) UUO mice significantly increased collagen contents to levels comparable to those in identically treated WT. Active TGF-beta protein expression in beta6(-/-) mice was less in UUO kidneys with or without Ang II infusion compared to matched WT mice. Activated Smad 2 levels in beta6(-/-) obstructed kidneys were lower than in WT UUO mice, and did not increase when fibrosis was induced in beta6(-/-) UUO mice by Ang II infusion. Anti-TGF-beta antibody only partially decreased this Ang II-stimulated fibrosis in beta6(-/-) UUO kidneys. In situ hybridization and immunostaining showed low expression of PAI-1 mRNA and protein in tubular epithelium in beta6(-/-) UUO kidneys, with increased PAI-1 expression in response to Ang II, Aldo, or both. Our results indicate that interruption of alphavbeta6-mediated activation of TGF-beta1 can protect against tubulointerstitial fibrosis. Further, the robust induction of tubulointerstitial fibrosis without increase in activated Smad 2 levels in obstructed beta6(-/-) mice by Ang II suggests the existence of a TGF-beta1-independent pathway of induction of fibrosis through angiotensin.
Figures





References
-
- Sharma K, Ziyadeh FN: The emerging role of transforming growth factor-beta in kidney diseases. Am J Physiol 1994, 266:F829-F842 - PubMed
-
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R: Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 1995, 108:2241-2251 - PubMed
-
- Arend LJ, Smart AM, Briggs JP: Mouse beta(6) integrin sequence, pattern of expression, and role in kidney development. J Am Soc Nephrol 2000, 11:2297-2305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

