Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes
- PMID: 14507638
- PMCID: PMC1868289
- DOI: 10.1016/S0002-9440(10)63488-8
Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes
Abstract
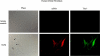
Fibroblasts represent a dynamic population of cells, exhibiting functional heterogeneity within and among tissues. Fibroblast heterogeneity also results from phenotypic differences and may arise from activation or differentiation processes taking place in the cells. We previously reported that human fibroblasts were heterogeneous with respect to surface Thy-1 expression and that separation into Thy-1(+) and Thy-1(-) subsets resulted in functionally distinct subpopulations, leading to the concept of fibroblast subset specialization. In this report we investigated whether Thy-1(+) and/or Thy-1(-) fibroblasts were capable of differentiating into myofibroblasts or lipofibroblasts. Fibroblast subsets were used from human myometrium and orbit to test this hypothesis. Only Thy-1(+) human myometrial and orbital fibroblasts were capable of myofibroblast differentiation after treatment with TGFbeta or platelet concentrate supernatant, assessed by alpha smooth muscle actin expression. Interestingly, only Thy-1(-), but not Thy-1(+) subsets differentiated to lipofibroblasts, as determined by the accumulation of cytoplasmic lipid droplets after treatment with 15-deoxy-Delta(12, 14)-PGJ(2) or ciglitazone. We propose that fibroblast Thy-1 display pre-determines lineage to a contractile or lipid-like phenotype in the human myometrium and orbit. This additional distinction between Thy-1(+) and Thy-1(-) human fibroblast subtypes has important consequences in normal tissue homeostasis and in pathogenesis of orbital and myometrial diseases characterized by persistent myofibroblasts or fat accumulation, such as occurs in Graves' ophthalmopathy, tissue fibrosis, abnormal wound healing, and scarring.
Figures





References
-
- Schneider EL, Mitsui Y, Au KS, Shorr SS: Tissue-specific differences in cultured human diploid fibroblasts. Exp Cell Res 1977, 108:1-6 - PubMed
-
- Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J: Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis 1988, 137:579-584 - PubMed
-
- Korn JH, Torres D, Downie E: Clonal heterogeneity in the fibroblast response to mononuclear cell derived mediators. Arthritis Rheum 1984, 27:174-179 - PubMed
-
- Botstein GR, Sherer GK, Leroy EC: Fibroblast selection in scleroderma: an alternative model of fibrosis. Arthritis Rheum 1982, 25:189-195 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

