Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy
- PMID: 14507653
- PMCID: PMC3278791
- DOI: 10.1016/S0002-9440(10)63503-1
Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy
Abstract
Complement activation during ischemia and reperfusion contributes to the development of tissue injury with severe negative impact on outcomes in transplantation. To counter the effect of complement, we present a strategy to deliver a novel complement regulator stabilized on cell surfaces within donor organs. The membrane-bound complement regulator is able to inhibit complement activation when the donor organ is revascularized and exposed to host-circulating complement. Application of this construct to donor kidneys protected transplanted tissues from ischemia/reperfusion injury and reduced the deposition of activated complement and histological signs of damage under conditions in which a nontargeted control construct was ineffective. Treatment of donor organs in this way improved graft performance in the short and long term. An analysis of the immune response in allograft recipients showed that reducing graft damage at the time of transplantation through complement regulation also modulated the alloresponse. Additionally, the results of perfusion studies with human kidneys demonstrated the feasibility of targeting endothelial and epithelial surfaces with this construct, to allow investigation in clinical transplantation.
Figures


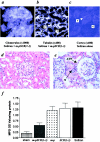



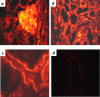
References
-
- Baldwin WM, Pruitt SK, Brauer RB, Daha MR, Sanfillipo F: Complement in organ transplantation: contributions to inflammation, injury and rejection. Transplantation 1995, 59:797-802 - PubMed
-
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT: Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 1990, 249:146-151 - PubMed
-
- Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD, Jr: Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol 1992, 149:1723-1728 - PubMed
-
- Mulligan MS, Yeh CG, Rudolph AR, Ward PA: Protective effects of soluble CR1 in complement and neutrophil-mediated tissue injury. J Immunol 1992, 148:1479-1486 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

