The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha
- PMID: 14507666
- PMCID: PMC1868300
- DOI: 10.1016/s0002-9440(10)63516-x
The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha
Abstract
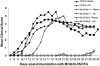
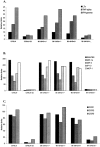
Low-dose estrogen (E2) treatment significantly inhibits the clinical signs and histopathological lesions of experimental autoimmune encephalomyelitis (EAE), and is being used in clinical trials to treat multiple sclerosis. To assess the role of intracytoplasmic estrogen receptors in mediating suppression of EAE, we studied mice with disrupted estrogen receptor-alpha (Esr1) and -beta (Esr2) genes. We demonstrate that the protective effect of E2 is abrogated in B6.129-Esr1(tm1Unc) mice (Esr1-/-) but not in B6.129-Esr2(tm1Unc) mice (Esr2-/-). The loss of E2-mediated protection from EAE in Esr1-/- mice immunized with the encephalitogenic MOG-35-55 peptide was manifested phenotypically by the development of severe acute clinical signs and histopathological lesions even in the presence of moderately high serum E2 levels. This is in contrast to C57BL/6 wild-type (WT) mice and Esr2-/- mice in which E2 treatment resulted in comparable serum levels and markedly suppressed clinical signs of EAE and abolished inflammatory lesions in the CNS. This pattern showing a lack of E2-dependent inhibition of EAE in Esr1-/- mice was mirrored by an enhanced rather than a reduced secretion of TNF-alpha, IFN-gamma, and interleukin (IL)-6 in MOG-specific splenocytes and a lack of inhibition of message for inflammatory cytokines, chemokines and chemokine receptors in CNS tissue. These results indicate that the immunomodulatory effects of E2 in EAE are dependent on Esr1 and not Esr2 signaling.
Figures

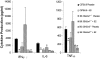
References
-
- Whitacre CC, Reingold SC, O’Looney PA, Blankenhorn E, Brinley F, Collier E, Duquette P, Fox H, Gilmore W, Lahita R, Nelson JL, Reiss C, Riskind P, Voskuhl RA: A gender gap in autoimmunity. Science 199, 283:1277-1278 - PubMed
-
- Jansson L, Holmdahl R: Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm Res 1998, 48:290-295 - PubMed
-
- Beeson PB: Age and sex association of 40 autoimmune diseases. Am J Med 1994, 96:457-462 - PubMed
-
- Birk K, Ford C, Meltzer S: The clinical course of multiple sclerosis during pregnancy during pregnancy and puerperium. Arch Neurol 1990, 47:738-742 - PubMed
-
- Korn-Lubetzki I, Kahana G, Cooper G, Abramsky O: Activity of multiple sclerosis during pregnancy and puerperium. Ann Neurol 1984, 16:229-231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

