Onset of DNA aggregation in presence of monovalent and multivalent counterions
- PMID: 14507678
- PMCID: PMC1303439
- DOI: 10.1016/S0006-3495(03)74638-4
Onset of DNA aggregation in presence of monovalent and multivalent counterions
Abstract
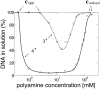
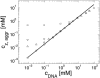
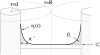
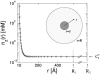
We address theoretically aggregation of DNA segments by multivalent polyamines such as spermine and spermidine. In experiments, the aggregation occurs above a certain threshold concentration of multivalent ions. We demonstrate that the dependence of this threshold on the concentration of DNA has a simple form. When the DNA concentration c(DNA) is smaller than the monovalent salt concentration, the threshold multivalent ion concentration depends linearly on c(DNA), having the form alphac(DNA) + beta. The coefficients alpha and beta are related to the density profile of multivalent counterions around isolated DNA chains, at the onset of their aggregation. This analysis agrees extremely well with recent detailed measurements on DNA aggregation in the presence of spermine. From the fit to the experimental data, the number of condensed multivalent counterions per DNA chain can be deduced. A few other conclusions can then be reached: 1), the number of condensed spermine ions at the onset of aggregation decreases with the addition of monovalent salt; 2), the Poisson-Boltzmann theory overestimates the number of condensed multivalent ions at high monovalent salt concentrations; and 3), our analysis of the data indicates that the DNA charge is not overcompensated by spermine at the onset of aggregation.
Figures
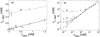



References
-
- Andelman, D. 1994. Electrostatic properties of membranes: the Poisson-Boltzmann theory. In Handbook of Physics of Biological Systems, Vol. I. R. Lipowsky, and E. Sackmann, editors. Elsevier Science, Amsterdam, pp.603–642.
-
- Arenzon, J. J., J. F. Stilck, and Y. Levin. 1999. Simple model for attraction between like-charged polyions. Eur. Phys. J. B. 12:79–82.
-
- Belloni, L., M. Drifford, and P. Turq. 1984. Counterion diffusion in polyelectrolyte solutions. Chem. Phys. 83:147–154.
-
- Bloomfield, V. A., D. M. Crothers, and I. Tinoco. 2000. Nucleic Acids: Structures, Properties, and Functions. University Science Books, Sausalito, CA.
-
- Borukhov, I., R. F. Bruinsma, W. M. Gelbart, and A. J. Liu. 2001. Elastically driven linker aggregation between two semiflexible polyelectrolytes. Phys. Rev. Lett. 86:2182–2185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

