Selective uptake of indocyanine green by reticulocytes in circulation
- PMID: 14507897
- PMCID: PMC2830078
- DOI: 10.1167/iovs.03-0041
Selective uptake of indocyanine green by reticulocytes in circulation
Abstract
Purpose: Hyperfluorescent cells labeled with indocyanine green (ICG) have been observed in retinal and choroidal circulation using scanning laser ophthalmoscopy. It has been suggested that ICG labels leukocytes and that ICG can be used to track leukocyte movement in vivo. The purpose of this study is to identify the cell population that takes up ICG and to study their trafficking pattern in vivo by confocal fluorescence microscopy.
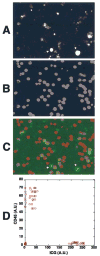
Methods: ICG was injected into the mouse tail vein, and images were taken by in vivo confocal microscopy. The trafficking pattern of ICG-labeled cells was compared with that of rhodamine 6G-labeled leukocytes. In vitro labeling of human blood cells with antibodies against cell lineage markers and with DNA stains was further used to identify the ICG-labeled cells. Antibodies against the following cell surface markers were used: CD45 (leukocytes), CD3 (T lymphocytes), CD19 (B lymphocytes), CD16 (Fc receptor), glycophorin A (erythroid lineage cells), and CD71 (transferrin receptor).
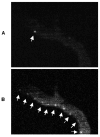
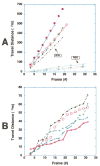
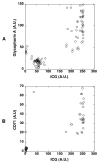
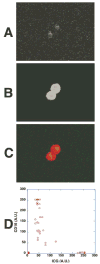
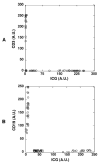
Results: The ICG-labeled cells were made up of two blood cell populations with distinct levels of ICG uptake. The strongly ICG-labeled cells did not roll on dermal vascular endothelium in vivo, in contrast to rhodamine 6G-labeled leukocytes. They were identified as reticulocytes because antibody staining showed that they were CD 45(-), glycophorin A(+) and CD 71(+). The weakly ICG-labeled cells were identified as neutrophils because they were CD45(+), CD16(+), CD3(-), and CD19(-).
Conclusions: ICG strongly labels reticulocytes and weakly labels neutrophils. To the authors' knowledge, this is the first report of selective staining of reticulocytes by ICG.
Conflict of interest statement
Disclosure:
Figures


References
-
- Slakter JS, Yannuzzi LA, Guyer DR, Sorenson JA, Orlock DA. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6:25–32. - PubMed
-
- Destro M, Puliafito CA. Indocyanine green videoangiography of choroidal neovascularization. Ophthalmology. 1989;96:846–853. - PubMed
-
- Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110:15–21. quiz 22-13. - PubMed
-
- Su MY, Lin DY, Sheen IS, Chu CM, Chiu CT, Liaw YF. Indocyanine green clearance test in non-cirrhotic hepatitis patients: a comparison and analysis between conventional blood sampling method and Finger Piece Monitoring method [in Chinese] Changgeng Yi Xue Za Zhi. 1999;22:17–23. - PubMed
-
- Ott P, Keiding S, Bass L. Intrinsic hepatic clearance of indocyanine green in the pig: dependence on plasma protein concentration. Eur J Clin Invest. 1992;22:347–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

