Postsynaptic protein kinase A reduces neuronal excitability in response to increased synaptic excitation in the Drosophila CNS
- PMID: 14507965
- PMCID: PMC6740429
- DOI: 10.1523/JNEUROSCI.23-25-08664.2003
Postsynaptic protein kinase A reduces neuronal excitability in response to increased synaptic excitation in the Drosophila CNS
Abstract
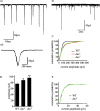
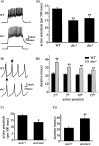
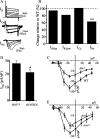
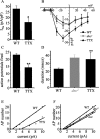
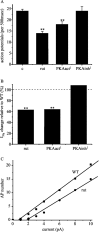
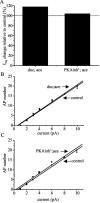
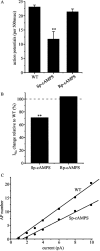
Previous work has identified a role for synaptic activity in the development of excitable properties of motoneurons in the Drosophila embryo. In this study the underlying mechanism that enables two such neurons, termed aCC and RP2, to respond to increased exposure to synaptic excitation is characterized. Synaptic excitation is increased in genetic backgrounds that lack either a cAMP-specific phosphodiesterase (EC:3.1.4, dunce) or acetylcholinesterase (EC:3.1.1.7, ace), the enzyme that terminates the endogenous cholinergic excitation of these motoneurons. Analysis of membrane excitability in aCC/RP2, in either background, shows that these neurons have a significantly reduced capability to fire action potentials (APs) in response to injection of depolarizing current. Analysis of underlying voltage-gated currents show that this effect is associated with a marked reduction in magnitude of the voltage-dependent inward Na+ current (INa). Partially blocking INa in these motoneurons, using low concentrations of TTX, demonstrates that a reduction of INa is, by itself, sufficient to reduce membrane excitability. An analysis of firing implicates an increased AP threshold to underlie the reduction in membrane excitability observed because of heightened exposure to synaptic excitation. Genetic or pharmacological manipulations that either elevate cAMP or increase protein kinase A (PKA) activity in wild-type aCC/RP2 mimic both the reductions in membrane excitability and INa. In comparison, increasing cAMP catabolism or inhibition of PKA activity is sufficient to block the induction of these activity-dependent changes. The induced changes in excitability can be rapid, occurring within 5 min of exposure to a membrane-permeable cAMP analog, indicative that threshold can be regulated in these neurons by a post-translational mechanism that is dependent on phosphorylation.
Figures
References
-
- Adelman JP, Shen K-Z, Kavanaugh MP, Warren RA, Wu Y-N, Lagrutta A, Bond CT, North RA ( 1992) Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9: 209-216. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B ( 1991) A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551-555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
