Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases
- PMID: 14507968
- PMCID: PMC6740426
- DOI: 10.1523/JNEUROSCI.23-25-08692.2003
Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases
Abstract
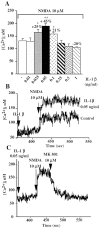
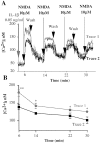
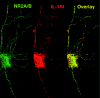
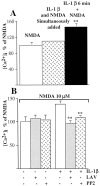
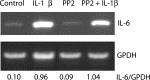
Interleukin (IL)-1beta is a proinflammatory cytokine implicated in various pathophysiological conditions of the CNS involving NMDA receptor activation. Circumstantial evidence suggests that IL-1beta and NMDA receptors can functionally interact. Using primary cultures of rat hippocampal neurons, we investigated whether IL-1beta affects NMDA receptor function(s) by studying (1) NMDA receptor-induced [Ca2+]i increase and (2) NMDA-mediated neurotoxicity. IL1beta (0.01-0.1 ng/ml) dose-dependently enhances NMDA-induced [Ca2+]i increases with a maximal effect of approximately 45%. This effect occurred only when neurons were pretreated with IL-1beta, whereas it was absent if IL-1beta and NMDA were applied simultaneously, and it was abolished by IL-1 receptor antagonist (50 ng/ml). Facilitation of NMDA-induced [Ca2+]i increase by IL-1beta was prevented by both lavendustin (LAV) A (500 nm) and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (1 microm), suggesting an involvement of tyrosine kinases. Increased tyrosine phosphorylation of NMDA receptor subunits 2A and 2B and coimmunoprecipitation of activated Src tyrosine kinase with these subunits was observed after exposure of hippocampal neurons to 0.05 ng/ml IL-1beta. Finally, 0.05 ng/ml IL-1beta increased by approximately 30% neuronal cell death induced by NMDA, and this effect was blocked by both lavendustin A and PP2. These data suggest that IL-1beta increases NMDA receptor function through activation of tyrosine kinases and subsequent NR2A/B subunit phosphorylation. These effects may contribute to glutamate-mediated neurodegeneration.
Figures




References
-
- Allan SM, Rothwell NJ ( 2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2: 734-744. - PubMed
-
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cul J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D ( 2002) Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415: 793-798. - PubMed
-
- Coogan A, O'Connor JJ ( 1997) Inhibition of NMDA receptor-mediated synaptic transmission in the rat dentate gyrus in vitro by IL-1 beta. NeuroReport 8: 2107-2110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
