ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes
- PMID: 14507996
- PMCID: PMC280565
- DOI: 10.1105/tpc.015560
ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes
Abstract
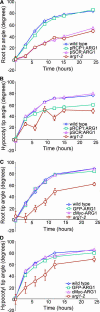
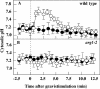
ARG1 (ALTERED RESPONSE TO GRAVITY) is required for normal root and hypocotyl gravitropism. Here, we show that targeting ARG1 to the gravity-perceiving cells of roots or hypocotyls is sufficient to rescue the gravitropic defects in the corresponding organs of arg1-2 null mutants. The cytosolic alkalinization of root cap columella cells that normally occurs very rapidly upon gravistimulation is lacking in arg1-2 mutants. Additionally, vertically grown arg1-2 roots appear to accumulate a greater amount of auxin in an expanded domain of the root cap compared with the wild type, and no detectable lateral auxin gradient develops across mutant root caps in response to gravistimulation. We also demonstrate that ARG1 is a peripheral membrane protein that may share some subcellular compartments in the vesicular trafficking pathway with PIN auxin efflux carriers. These data support our hypothesis that ARG1 is involved early in gravitropic signal transduction within the gravity-perceiving cells, where it influences pH changes and auxin distribution. We propose that ARG1 affects the localization and/or activity of PIN or other proteins involved in lateral auxin transport.
Figures




References
-
- Aguilar, R.M., Bustamante, J.J., Hernandez, P.G., Martinez, A.O., and Haro, L.S. (1999). Precipitation of dilute chromatographic samples (ng/ml) containing interfering substances for SDS-PAGE. Anal. Biochem. 267, 344–350. - PubMed
-
- Artigues, A., Iriarte, A., and Martinez-Carrion, M. (2002). Binding to chaperones allows import of a purified mitochondrial precursor into mitochondria. J. Biol. Chem. 277, 25047–25055. - PubMed
-
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273, 948–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

