Selective regulation of CD40 expression in murine dendritic cells by thiol antioxidants
- PMID: 14511233
- PMCID: PMC1783048
- DOI: 10.1046/j.1365-2567.2003.01723.x
Selective regulation of CD40 expression in murine dendritic cells by thiol antioxidants
Abstract
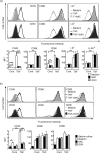
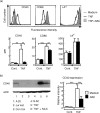
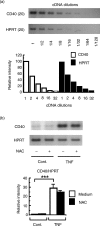
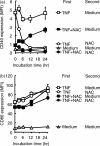
Interaction of CD40 on dendritic cells (DC) with CD40 ligand induces interleukin-12 (IL-12) production by these DC during the antigen presentation. Thus, the level of CD40 expression appears to influence the capability of DC to induce a T helper 1 (Th1) response. However, it is not fully understood how CD40 expression on DC is regulated. In the present study, we examined the effects of the reducing agents, N-acetyl-l-cysteine (NAC) and reduced glutathione (GSH), on tumour necrosis factor-alpha (TNF-alpha)-induced phenotypic changes in murine DC. TNF-alpha markedly increased the expression on DC of major histocompatibility complex (MHC) and the costimulatory molecules, CD40, CD80 and CD86. Both NAC and GSH completely abolished the TNF-alpha-induced enhancement of CD40 expression, but had no considerable effect on the expression of CD80, CD86 and MHC. The marked decrease of CD40 protein with NAC was also detected by Western blotting, but was not associated with the expression level of CD40 mRNA in DC. Thus, NAC appears to reduce CD40 expression on DC by regulating a post-transcriptional pathway. The inhibitory effect of NAC or GSH on TNF-alpha-induced CD40 expression was released by simply removing these agents from the culture. In contrast, culture of TNF-alpha-treated DC with NAC or GSH markedly decreased the expression of CD40 within 12 hr. These results demonstrate that reducing agents selectively, rapidly and reversibly regulate CD40 expression on DC, which may eventually affect the capability of DC for Th1/Th2 polarization.
Figures

References
-
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Lane PJ, Brocker T. Developmental regulation of dendritic cell function. Curr Opin Immunol. 1999;11:308–13. - PubMed
-
- Lipscomb MF, Masten BJ. Dendritic cells. immune regulators in health and disease. Physiol Rev. 2002;82:97–130. - PubMed
-
- Van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol. 2000;67:2–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

