Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway
- PMID: 14512515
- PMCID: PMC218758
- DOI: 10.1073/pnas.1933740100
Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway
Abstract
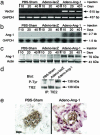
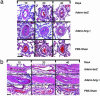
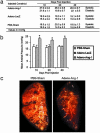
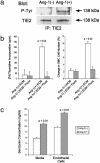
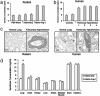
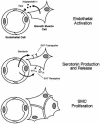
Smooth muscle cell proliferation around small pulmonary vessels is essential to the pathogenesis of pulmonary hypertension. Here we describe a molecular mechanism and animal model for this vascular pathology. Rodents engineered to express angiopoietin 1 (Ang-1) constitutively in the lung develop severe pulmonary hypertension. These animals manifest diffuse medial thickening in small pulmonary vessels, resulting from smooth muscle cell hyperplasia. This pathology is common to all forms of human pulmonary hypertension. We demonstrate that Ang-1 stimulates pulmonary arteriolar endothelial cells through a TIE2 (receptor with tyrosine kinase activity containing IgG-like loops and epidermal growth factor homology domains) pathway to produce and secrete serotonin (5-hydroxytryptamine), a potent smooth muscle mitogen, and find that high levels of serotonin are present both in human and rodent pulmonary hypertensive lung tissue. These results suggest that pulmonary hypertensive vasculopathy occurs through an Ang-1/TIE2/serotonin paracrine pathway and imply that these signaling molecules may be targets for strategies to treat this disease.
Figures
References
-
- Folkman, J. & D'Amore, P. A. (1996) Cell 87, 1153–1155. - PubMed
-
- Hayes, A. J., Huang, W. Q., Mallah, J., Yang, D., Lippman, M. E. & Li, L. Y. (1999) Microvasc. Res. 58, 224–237. - PubMed
-
- Davis, S., Aldrich, T. H., Jones, P. F., Acheson, A., Compton, D. L., Jain, V., Ryan, T. E., Bruno, J., Radziejewski, C., Maisonpierre, P. C., et al. (1996) Cell 87, 1161–1169. - PubMed
-
- Jones, N. & Dumont, D. J. (2000) Cancer Metastasis Rev. 19, 13–17. - PubMed
-
- Davis, S. & Yancopoulos, G. D. (1999) Curr. Top. Microbiol. Immunol. 237, 173–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

