Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years
- PMID: 14512569
- PMCID: PMC224988
- DOI: 10.1128/jvi.77.20.11212-11219.2003
Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years
Abstract
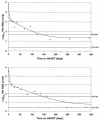
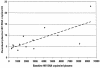
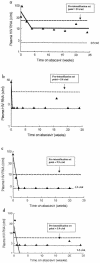
To provide insight into the dynamics and source of residual viremia in human immunodeficiency virus (HIV) patients successfully treated with antiretroviral therapy, 14 intensely monitored patients treated with indinavir and efavirenz sustaining HIV RNA at <50 copies/ml for >5 years were studied. Abacavir was added to the regimen of eight patients at year 5. After the first 9 months of therapy, HIV RNA levels had reached a plateau ("residual viremia") that persisted for over 5 years. Levels of residual viremia differed among patients and ranged from 3.2 to 23 HIV RNA copies/ml. Baseline HIV DNA was the only significant pretreatment predictor of residual viremia in regression models including baseline HIV RNA, CD4 count, and patient age. In the four of five patients with detectable viremia who added abacavir to their regimen after 5 years, HIV RNA levels declined rapidly. The estimated half-life of infected cells was 6.7 days. Decrease in activated memory cells and a reduction in gamma interferon production to HIV Gag and p24 antigen in ELISpot assays were observed, consistent with a decrease in HIV replication. Thus, in patients treated with efavirenz plus indinavir, levels of residual viremia were established by 9 months, were predicted by baseline proviral DNA, and remained constant for 5 years. Even after years of highly suppressive therapy, HIV RNA levels declined rapidly after the addition of abacavir, suggesting that productive infection contributes to residual ongoing viremia and can be inhibited with therapy intensification.
Figures

References
-
- Andreoni, M., S. G. Parisi, L. Sarmati, E. Nicastri, L. Ercoli, G. Mancino, G. Sotgiu, M. Mannazzu, M. Trevenzoli, G. Tridente, E. Concia, and A. Aceti. 2000. Cellular proviral HIV-DNA decline and viral isolation in naive subjects with <5,000 copies/ml of HIV-RNA and >500 × 106/l CD4 cells treated with highly active antiretroviral therapy. AIDS 14:23-29. - PubMed
-
- Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Blankson, J. N., D. Finzi, T. C. Pierson, B. P. Sabundayo, K. Chadwick, J. B. Margolick, T. C. Quinn, and R. F. Siciliano. 2000. Biphasic decay of latently infected cD4+ T cells in acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:1636-1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

