Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF
- PMID: 14514885
- PMCID: PMC218784
- DOI: 10.1073/pnas.1934836100
Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF
Abstract
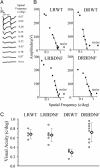
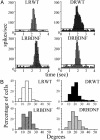
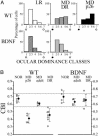
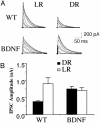
Visual deprivation such as dark rearing (DR) prolongs the critical period for ocular dominance plasticity and retards the maturation of gamma-aminobutyric acid (GABA)ergic inhibition in visual cortex. The molecular signals that mediate the effects of DR on the development of visual cortex are not well defined. To test the role of brain-derived neurotrophic factor (BDNF), we examined the effects of DR in transgenic mice in which BDNF expression in visual cortex was uncoupled from visual experience and remained elevated during DR. In dark-reared transgenic mice, visual acuity, receptive field size of visual cortical neurons, critical period for ocular dominance plasticity, and intracortical inhibition were indistinguishable from those observed in light-reared mice. Therefore, BDNF overexpression is sufficient for the development of aspects of visual cortex in the absence of visual experience. These results suggest that reduced BDNF expression contributes to retarded maturation of GABAergic inhibition and delayed development of visual cortex during visual deprivation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

