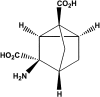
WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake
- PMID: 14517179
- PMCID: PMC1574101
- DOI: 10.1038/sj.bjp.0705509
WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake
Abstract
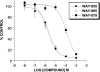
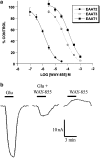
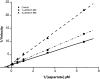
The pharmacological profile of a novel glutamate transport inhibitor, WAY-855 (3-amino-tricyclo[2.2.1.0(2.6)]heptane-1,3-dicarboxylic acid), on the activity of the human forebrain glutamate transporters EAAT1, EAAT2 and EAAT3 expressed in stable mammalian cell lines and in Xenopus laevis oocytes is presented. WAY-855 inhibited glutamate uptake mediated by all three subtypes in a concentration-dependent manner, with preferential inhibition of the CNS-predominant EAAT2 subtype in both cells and oocytes. IC50 values for EAAT2 and EAAT3 inhibition in cells were 2.2 and 24.5 microM, respectively, while EAAT1 activity was inhibited by 50% at 100 microM (IC50 values determined in oocytes were 1.3 microM (EAAT2), 52.5 microM (EAAT3) and 125.9 microM (EAAT1)). Application of WAY-855 to EAAT-expressing oocytes failed to induce a transporter current, and the compound failed to exchange with accumulated [3H]d-aspartate in synaptosomes consistent with a nonsubstrate inhibitor. WAY-855 inhibited d-aspartate uptake into cortical synaptosomes by a competitive mechanism, and with similar potency to that observed for the cloned EAAT2. WAY-855 failed to agonise or antagonise ionotropic glutamate receptors in cultured hippocampal neurones, or the human metabotropic glutamate receptor subtype 4 expressed in a stable cell line. WAY-855 represents a novel structure in glutamate transporter pharmacology, and exploration of this structure might provide insights into the discrimination between EAAT2 and other EAAT subtypes.
Figures




References
-
- BARNARD E.A. Ionotropic glutamate receptors: new types and new concepts. Trends Pharmacol. Sci. 1997;18:141–148. - PubMed
-
- BERGLES D.E., JAHR C.E. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. - PubMed
-
- BRIDGES R.J., KAVANAUGH M.P., CHAMBERLIN A.R. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr. Pharmaceutical Des. 1999;5:363–379. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases

