Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44
- PMID: 14517240
- PMCID: PMC204474
- DOI: 10.1093/emboj/cdg491
Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44
Abstract
Formation of disulfide bonds, an essential step for the maturation and exit of secretory proteins from the endoplasmic reticulum (ER), is controlled by specific ER-resident enzymes. A pivotal element in this process is Ero1alpha, an oxidoreductin that lacks known ER retention motifs. Here we show that ERp44 mediates Ero1alpha ER localization through the formation of reversible mixed disulfides. ERp44 also prevents the secretion of an unassembled cargo protein with unpaired cysteines. We conclude that ERp44 is a key element in thiol-mediated retention. It might also favour the maturation of disulfide-linked oligomeric proteins and their quality control.
Figures

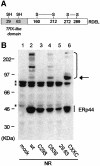
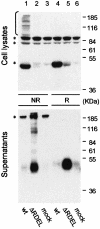

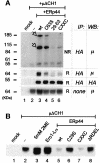
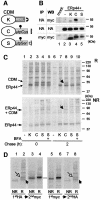
References
-
- Alberini C.M., Bet,P., Milstein,C. and Sitia,R. (1990) Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature, 347, 485–487. - PubMed
-
- Benedetti C., Fabbri,M., Sitia,R. and Cabibbo,A. (2000) Aspects of gene regulation during the UPR in human cells. Biochem. Biophys. Res. Commun., 278, 530–536. - PubMed
-
- Cabibbo A., Pagani,M., Fabbri,M., Rocchi,M., Farmery,M.R., Bulleid,N.J. and Sitia,R. (2000) ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem., 275, 4827–4833. - PubMed
-
- Ellgaard L. and Helenius,A. (2001) ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol., 13, 431–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

