Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo
- PMID: 14517254
- PMCID: PMC204462
- DOI: 10.1093/emboj/cdg478
Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo
Abstract
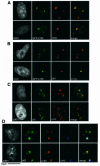
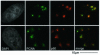
DNA damage and its repair can cause both local and global rearrangements of chromatin structure. In each case, the epigenetic information contained within this structure must be maintained. Using the recently developed method for the localized UV irradiation of cells, we analysed responses that occur locally to damage sites and global events triggered by local damage recognition. We thus demonstrate that, within a single cell, the recruitment of chromatin assembly factor 1 (CAF-1) to UV-induced DNA damage is a strictly local phenomenon, restricted to damage sites. Concomitantly, proliferating cell nuclear antigen (PCNA) locates to the same sites. This localized recruitment suggests that CAF-1 participates directly in chromatin structural rearrangements that occur in the vicinity of the damage. Use of nucleotide excision repair (NER)-deficient cells shows that the NER pathway--specifically dual incision--is required for recruitment of CAF-1 and PCNA. This in vivo demonstration of the local role of CAF-1, depending directly on NER, supports the hypothesis that CAF-1 ensures the maintenance of epigenetic information by acting locally at repair sites.
Figures






References
-
- Aboussekhra A. and Wood,R.D. (1995) Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Exp. Cell Res., 221, 326–332. - PubMed
-
- Bakkenist C.J. and Kastan,M.B. (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature, 421, 499–506. - PubMed
-
- Batty D.P. and Wood,R.D. (2000) Damage recognition in nucleotide excision repair of DNA. Gene, 241, 193–204. - PubMed
-
- Bernard P., Maure,J.F., Partridge,J.F., Genier,S., Javerzat,J.P. and Allshire,R.C. (2001) Requirement of heterochromatin for cohesion at centromeres. Science, 294, 2539–2542. - PubMed
-
- Biggerstaff M. and Wood,R.D. (1999) Assay for nucleotide excision repair protein activity using fractionated cell extracts and UV-damaged plasmid DNA. Methods Mol. Biol., 113, 357–372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

